Description
- “Blood substitutes” have been studied for >50 years and are either derivatives of hemoglobin or perfluorocarbons; they are designed to carry and offload oxygen to tissues.
- No products discussed are FDA approved for human use. One product (Hemopure®, OPK Biotech, Cambridge, MA) has been approved in South Africa and Russia. Another product (Oxyglobin®, OPK Biotech) is FDA and European Union approved for canine anemia.
- Blood substitutes have been designed to carry oxygen to ischemic tissue as well as tested as a resuscitation fluid.
- HBOCs are:
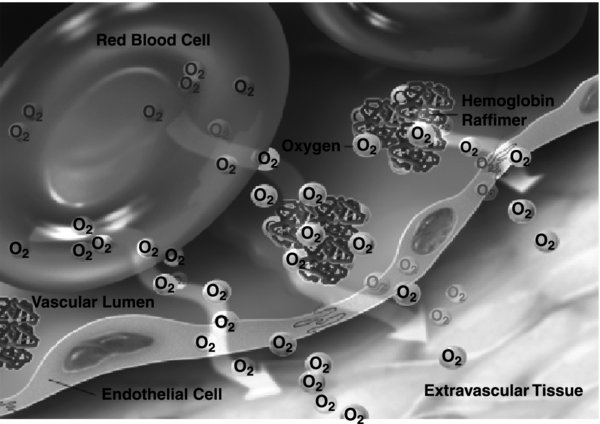
- Synthesized from human or bovine blood cells in a process that removes the red cell membrane, purifies and deactivates pathogens (prions), and re-polymerizes the purified hemoglobin (via glutaraldehyde pegylation, encapsulation, or zero-link polymerization).
- Prepared as a bag of solution around 250–500 mL, or the equivalent amount of hemoglobin as a unit of packed red blood cells. Some of the HBOCs offload oxygen more easily and may be more efficient than banked blood.
- Newer generations have attempted to link oxygen carrying with diminished extravasation from the vascular compartment, block nitric oxide scavenging, and serve as anti-inflammatory agents to diminish the deleterious effects of ischemia.
- “First-generation” HBOCs are designed to have a normal hemoglobin (10–13 g/dL), normal viscosity, elevated colloid oncotic pressure, shift the oxyhemoglobin dissociation curve to the right, and a normal Hill coefficient.
- They include
 -
- cross-linked hemoglobin, 2,3 diaspirin cross-linked hemoglobin (HemAssist®, Baxter, Deerfield, IL), and hemoglobin raffimer (Hemolink™, Hemosol, Toronto, ON).
cross-linked hemoglobin, 2,3 diaspirin cross-linked hemoglobin (HemAssist®, Baxter, Deerfield, IL), and hemoglobin raffimer (Hemolink™, Hemosol, Toronto, ON).
- They include
- “Second-generation” HBOCs are designed with the same goals as first-generation HBOCs but with fewer side effects. They too have a normal hemoglobin (10–13 g/dL), normal viscosity, elevated colloid oncotic pressure, shift the oxyhemoglobin dissociation curve to the right, and a normal Hill coefficient. To date, they remain the most successful HBOCs.
- Human polyhemoglobin (PolyHeme®, Northfield, Evanston, IL) and hemoglobin glutamer (bovine) 200 and 250 (Hemopure® and Oxyglobin®, OPK Biotech) have completed FDA Phase III testing but were not approved in the US.
- “Third-generation” HBOCs have a low hemoglobin (5–6 g/dL), normal to high viscosity, lower colloid oncotic pressure, shift the oxyhemoglobin dissociation curve to the left, and a normal Hill coefficient.
- There are a number of “third-generation” HBOCs currently undergoing preclinical and clinical testing (Maleimide-Polyethylene Glyco-modified Hemoglobin [MP4], Hemospan®, Sangart, San Diego, CA; Zero-linked Hemoglobin Polymer Oxyvita®, OXYVITA, New Windsor, NY).
Physiology/Pathophysiology
- “First-generation” HBOCs had serious complications including renal failure and increased mortality in trauma trials (HemAssist®).
- “Second-generation” HBOCs cause increased systemic and pulmonary blood pressure, increased lipase without clinical signs or symptoms of pancreatitis, and transient jaundice, secondary to breakdown of the hemoglobin by the reticuloendothelial system.
- “Third-generation” HBOCs may avoid the hypertension, but may still have other complications.
- In one study, elderly patients tended to have worse outcomes with the particular blood substitute, suggesting that patients with pre-existing hypertension, cardiac, renal, and cerebrovascular disease may be susceptible to the hypertensive effects.
- The concept of blood substitutes is attractive in that they are:
- Stable at room temperature
- Do not require cross-matching and are immediately available
- Do not carry infectious disease risk
- Not dependent on a limited donor supply
- Possible indications for blood substitutes include:
- Shock
- Organ ischemia
- Red blood cell incompatibility
- Acute lung injury
- Transplant organ preservation
- Cardioplegia
- Sickle cell anemia
- Tumor therapy
- Air embolism
- Anemia refractory to allogenic blood transfusion
- Emergency civilian or military setting of severe trauma or perioperative bleeding
- Allogenic blood transfusions have multiple risks, including infectious, immunologic, metabolic, and critical illnesses.
- Screening of blood has reduced the incidence of HIV and Hepatitis C; however, new infections take time to be detected and removed from the donor supply.
- Immunomodulation can result in increased surgical wound infection and recurrence of cancer (especially colon cancer) as well as decreased graft survival of transplanted organs.
- Transfusion-related acute lung injury (TRALI) can occur in 1:5,000 transfusions and carries a high mortality ranging from 6% to 9%.
- Transfusion reactions from laboratory or transfusion error can result in serious morbidity and mortality.
- Metabolic derangements include hypothermia, hyperkalemia, and decreased 2,3-DPG.
- Transfusion-associated circulatory overload (TACO) is due to rapid transfusion of a large volume of blood and can cause dyspnea, orthopnea, peripheral edema, and rapid increases in blood pressure. It carries an incidence of 1:100–10,000.
- However, even if a blood substitute is developed and has minimal complications, blood donation cannot be replaced; donor blood is fractionated to multiple products including platelets, fresh frozen plasma, and cryoprecipitate.