Description
- The raw EEG is a reflection of cortical brain activity.
- EEG monitoring may be utilized in the perioperative period to assess the depth of anesthesia and attainment of burst suppression, as well as indirectly monitor for cortical ischemia during surgical procedures (aneurysm clipping, carotid endarterectomy).
- Analysis of the EEG requires knowledge of the differing waveforms. However, the complexity can mislead even sophisticated quantitative monitors and can lead to erroneous clinical decisions.
- Modern monitors, such as the Bispectral Index (BIS) and Spectral Entropy (M-entropy), attempt to quantify this raw EEG, yielding an easy-to-interpret number.
- The EEG is influenced not only by the medications administered, but also by a myriad of factors in the patient's physiology and underlying pathology.
- An accurate assessment of the depth of anesthesia via the raw EEG may reduce the incidence of awareness and improve recovery from anesthesia.
- Classically, patients require a full "montage" of electrodes covering the entire scalp to capture the whole spectrum of EEG generated. This is especially true when looking for regional ischemia (e.g., during carotid endarterectomy, and clipping of cerebral aneurysms) due to surgical interventions to guide further surgical decisions.
- for the purpose of depth of anesthesia, monitoring 3 or 4 electrodes placed on the forehead close to the hairline will obtain an EEG sufficient to assess the depth of anesthesia (positive, negative, and reference). The rest of the scalp may be "neglected" since the frontal aspect is more resistant to pharmacologic sleep than the more posterior aspects (frontal predominance); allows for a margin of safety.
- The surface electrodes pick up voltage fluctuations in the synaptic activity of the dendrites, and the sum of a large number of these is reflected in each EEG electrode. They are not specific to the voltage generated by dendritic cells, and will pick up signals generated by other, unwanted current sources such as facial, eye, and heart muscles, and electrical sources. The understanding of these noise generators is crucial in making clinical decisions.
- Myogenic activity can be distinguished from the EEG by causing the waveform to "wander" above and below the baseline. Patient blinking and facial movement will cause a similar deviation from baseline and will make identification of these noise generators easy.
- Muscle relaxants will eliminate the myogenic artifact. However, one must be careful to administer paralytics to patients who are only marginally asleep to avoid recall. Studies have shown that, even in the absence of myogenic artifact, the EEG is depressed by administering paralytics. This is likely due to reduction in the afferent input to the brain from muscle spindles, slowing the EEG.
- Electrical interference from line currents have a frequency of 60 Hz and are easy to detect; the highest frequency of EEG encountered is typically 30 Hz
- EEG patterns (see Table 1).
- Awake: High frequency and low voltage; the tracing will appear as a blurred line on the monitor.
- Sedation: Loss of fast
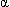 activity and an increase in
activity and an increase in  activity.
activity. - Deep sedation: Slowing of
 activity, appearance of spindle activity. Eventually,
activity, appearance of spindle activity. Eventually, 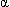 and
and  activity disappears, and
activity disappears, and  activity appears.
activity appears. - General anesthesia:
 waves predominate.
waves predominate. 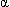 and
and  activity disappear, and with increasing depth, slow waves (
activity disappear, and with increasing depth, slow waves ( ) predominate.
) predominate. - Deep levels of general anesthesia: Isoelectric EEG interrupted by "bursts" of electrical activity (burst suppression).
- Further deepening of anesthesia: Increase the length spent suppressed and reduce the number of "bursts." Eventually, isoelectricity will prevail without "bursts."
- Characteristics may not be easily evident with rapid transition between phases (i.e., during induction of general anesthesia)
- Physiologic sleep and anesthetic sleep are similar, but not identical
- Different anesthetic agents will produce different EEG patterns.
- Physiologic sleep and anesthetic sleep are similar, but not identical. Different anesthetic agents will produce different EEG patterns.
- Extrapyramidal neurons are arranged in the cerebral cortex.
- Synaptic activity in the dendrites causes electrical activity, which changes over time and place.
- These fluctuations in potentials in the dendritic trees of pyramidal neurons are summed up by overlying electrodes on the scalp.
Physiology/Pathophysiology
- Baseline EEGs in the awake patient aid with establishing "normal" and differentiating pathology intraoperatively.
- Low-amplitude EEG can be drug induced, genetically determined, pathologic, or metabolic (e.g., hypoglycemia).
- Healthy patients in an awake state can demonstrate very slow EEGs.
- Dementia patients may display increased slow wave activity and decreased fast activity.
- Schizophrenics have an increase in frontal slow waves, and a decrease in
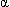 waves.
waves. - Alcohol generally increases
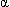 waves, and severe intoxication can cause burst suppression.
waves, and severe intoxication can cause burst suppression. - Cocaine and cannabis increase
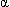 activity.
activity. - Cerebrovascular disease causes slowing.
- Severe brain injury and cerebral palsy cause significant EEG slowing.
- Cerebral ischemia. EEG monitoring to detect cerebral ischemia (e.g. during carotid endarterectomy, aneurysm clipping) is well established. Slowing of the EEG during a stable anesthetic and stable surgical stimulation should alert one to cerebral ischemia if the blood pressure is marginal.
- Changes in EEG pattern: Hypoglycemia, hypothermia, and hypotension will cause EEG slowing and even progress to an isoelectric EEG
 pattern
pattern - There may be periods of time where despite a stable level of anesthesia and no change in surgical stimulation, patients will transition to a predominately
 pattern, indicating a very deep level of anesthesia. The cause of this is unknown, and the significance of this is also not known. One must be vigilant and pursue possible causes for this (e.g., cerebral ischemia) if this pattern persists despite lightening of anesthetic depth.
pattern, indicating a very deep level of anesthesia. The cause of this is unknown, and the significance of this is also not known. One must be vigilant and pursue possible causes for this (e.g., cerebral ischemia) if this pattern persists despite lightening of anesthetic depth. - May paradoxically appear during sudden noxious stimuli; the cause remains unknown. This appearance may give the appearance of actually increasing anesthetic depth, yet the patient is actually transitioning into a lighter plane of anesthesia.
- There may be periods of time where despite a stable level of anesthesia and no change in surgical stimulation, patients will transition to a predominately
- The EEG can be used as a gauge of anesthetic depth and possibly reduce the risk of awareness as well as improve the recovery from anesthesia, and identify risk of cerebral ischemia.
- The EEG can be used as an indicator of cerebral perfusion (e.g., during carotid endarterectomy, cerebral aneurysm clipping). Changes to look for would be a sudden slowing or isoelectricity with clamp application.
- The EEG is a poor predictor of movement. There are several reasons for this. The EEG reflects cortical activity, and movement during anesthesia is not a cortical phenomenon, but controlled at deeper levels of the brain, and also at the level of the spinal cord. A patient can be judged to be "deep" yet move. The sequence of events will be that the EEG is deep, a noxious stimulus is administered to the patient, generators at the spinal cord or deep brain level will initiate patient movement, and eventually these stimuli will transition to the cortical level. The EEG will display a "lighter" level of anesthesia AFTER the patient has moved. If the brain activity is recorded in deeper structures (such as near the thalamus) a much better correlation between depth and patient movement can be obtained.
- The EEG is useful in situations where patients are at greatest risk for awareness and recall. Groups at risk are traditionally trauma patients, women having emergent Cesarean sections, and patients undergoing cardiac surgery. In these scenarios, either because of the intolerance to adequate anesthesia, or the urgency for a rapid induction, patients may be at higher risk for awareness. The EEG can be an adjunct to other parameters correlating with patient depth (blink reflex, movement, blood pressure, heart rate, etc.) (3).
- Barbiturates initially produce fast EEG waves, and then successively slower waves culminating in isoelectricity with increasing doses. The initial "rapid response" is only seen if very small doses are given. It is not seen with typical induction doses.
- Isoflurane at subanesthetic doses will yield fast activation, and slowing of the EEG with increasing concentration. Isoflurane can produce isoelectricity.
- Sevoflurane is very similar to isoflurane in its effect on the EEG, but has been observed to cause seizure-like movements at high concentrations.
- Ketamine, an NMDA receptor antagonist, causes an increase in the frequency of the EEG. This may mislead the practitioner to erroneously interpret this as lighter anesthesia, and overdose the patient. This will also lead to higher numbers on quantitative EEG monitors such as the BIS.
- Nitrous oxide has a minimal effect on the EEG. Therefore, a patient on a nitrous oxide–volatile anesthetic can actually be deeper than a patient who is on a volatile anesthetic alone despite the fact that the EEG indicates the patient to be more lightly anesthetized. Nitrous oxide may also cause a decrease in the frequency of the EEG several minutes after discontinuation.
- Opioids in small doses have a minimal effect on the EEG, but produce slowing at larger doses. Opioids will either slow an EEG or reduce the likelihood of movement at a stable EEG. Alfentanil may produce seizure-like activity, or unmask epileptiform foci.
- Epinephrine and ephedrine can cause increasing frequencies on the EEG.
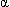 -2 agonists, such as clonidine and dexmedetomidine, have similar effects on the EEG as barbiturates, propofol, and etomidate.
-2 agonists, such as clonidine and dexmedetomidine, have similar effects on the EEG as barbiturates, propofol, and etomidate.