Description
- Anaerobic metabolism is the body's mechanism of continually responding to stress and sustaining life-preserving measures during malperfusion, until favorable conditions are resumed.
- The human body's major source of energy comes from the consumption of glucose via aerobic methods:
- Aerobic metabolism: Occurs when there is an adequate delivery of oxygen. The O2 molecule functions as an electron acceptor to produce a maximal amount of energy in the form of ATP (adenosine triphosphate).
- Anaerobic metabolism: Occurs during times of malperfusion and oxygen supply–demand mismatch. The body generates ATP via a rapid, oxygen-free variation of glycolysis with lactate as a byproduct (the Pasteur Effect).
- ATP is a nucleotide that provides chemical energy for all cells in the body; it is produced via carbohydrate (primarily) and non-carbohydrate metabolism.
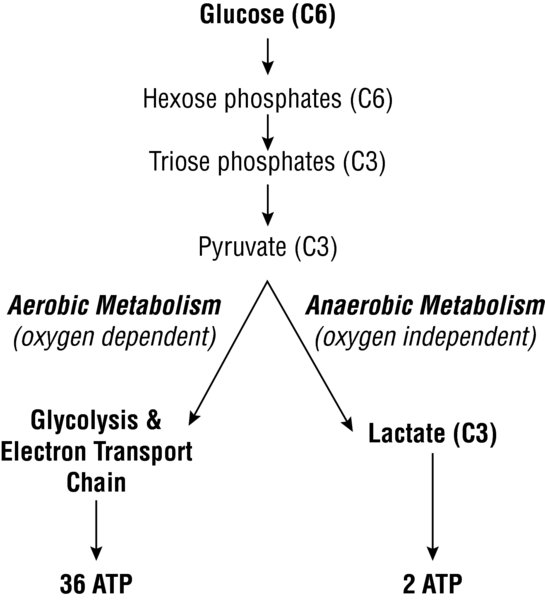
- Aerobic metabolism (oxygen-dependent) occurs via metabolism of glucose within the cytoplasm and mitochondria; each molecule of glucose is capable of producing 36 ATP.
- Glycolysis: Glucose is a 6-carbon structure that is first broken down into pyruvate, a 3-carbon structure. This process takes place in the cytoplasm and results in the net production of 2 ATP.
- Citric acid cycle (Krebs' cycle): Using the pyruvate molecules and available oxygen, the citric acid cycle generates 2 additional ATP per original glucose molecule via an 8-step process and 18 different enzymes. In addition, this also generates NADH, FADH2, and other byproducts (e.g., GTP) that can be converted to ATP.
- Oxidative phosphorylation: NADH and FADH2 are metabolized in the mitochondria to create an additional 24 molecules of ATP per original glucose molecule. This occurs via oxidative phosphorylation (the electron transport chain), driven by a proton gradient.
- Gluconeogenesis: In the absence of readily available supplies of glucose, the body is able to use non-carbohydrates (e.g., lactate, glycerol, and certain amino acids or fatty acids) to generate glucose. Gluconeogenesis takes places via enzymes found in the cytoplasm and mitochondria of hepatic cells; liver dysfunction or failure can, thus, result in hypoglycemia and require supplemental dextrose solutions.
- Glycogenolysis: The body can also convert complex carbohydrates, such as glycogen, to glucose. This pathway occurs in the liver and muscle; it also requires a functioning liver.
- Anaerobic metabolism (oxygen-independent) occurs when the demand for oxygen is greater than its supply. for each glucose molecule, only 2 ATP are produced.
- In states where oxygen is reduced/absent, pyruva te is metabolized to lactate via lactate dehydrogenase as an end-product of anaerobic metabolism .
- Physiologic states (exercise): The anaerobic threshold is a theoretical point during dynamic exercise when muscle tissue switches over to anaerobic metabolism as an additional energy source.
- Pathologic states: Stress, hypoxia, or hypotension/shock
- Central nervous system: The CNS consumes large amounts of energy to maintain normal electrical function and cellular metabolism.
- Because it cannot store ATP, it requires a constant supply of both glucose and oxygen (~3–3.5 mLO2/100 g/min).
- Anaerobic metabolism can result in a 95% decrease in energy production per available glucose. This can further worsen ischemic damage.
- It is not capable of utilizing amino acids or fatty acids as alternative sources of energy. Thus, hypoxia, hypoglycemia, or impaired perfusion can rapidly lead to inadequate energy levels.
- Myocardium: Due to its demand for a constant source of energy, cardiac muscle is highly dependent on the aerobic metabolism of fats, carbohydrates, and amino acids. Under ischemic or hypoxic conditions, the energy liberated by lactate production or anaerobic metabolism (as compared to aerobic metabolism) is not sufficient for myocardial function and ventricular contraction.
- Pulmonary: Lactic acidosis stimulates peripheral and central chemoreceptors to augment the medullary respiratory center. This leads to a compensatory increase in oxygen intake and excretion of carbon dioxide. As with any change in acid–base homeostasis, the ventilatory response to acidosis is driven primarily by central chemoreceptors; whereas peripheral chemoreceptors are primarily affected by hypoxemia.
- Hemoglobin: Acidosis decreases the affinity of hemoglobin for oxygen; it improves oxygen uploading/delivery to acidotic tissue. This is expressed as a right shift in the oxygen–hemoglobin dissociation curve.
Physiology/Pathophysiology
- Lactate accumulation occurs during anaerobic metabolism and results in an anion gap metabolic acidosis.
- An anion gap is a term used to describe "unmeasured anions" (e.g., lactate, ethanol, uremia, certain toxins, ketones) during states of metabolic acidosis.
- Normal lactate concentrations range from 0.5 to 1.0 mmol/L in healthy individuals and up to 2.0 mmol/L in the critically ill.
- Base excess is more commonly measured in the perioperative period.
- Perioperative lactic acidosis indicates tissue hypoxia from any combination of the following:
- Hypovolemia (e.g., hemorrhagic shock in a trauma patient)
- Hypoperfusion (e.g., cardiogenic shock following cardiac surgery or myocardial ischemic events)
- Hypotension (e.g., vasodilatory shock in a septic patient)
- Multifactorial from a combination of the above. for example, a trauma patient suffering myocardial contusions and a perforated viscus could have a combination of hypovolemia, hypoperfusion, and hypotension.
- Lactic acidosis leads to several cellular and microvascular changes in tissues (1).
- Hyperlactatemia can result from:
- Increased lactate production (Type A): Due to anaerobic metabolism
- Decreased lactate clearance (Type B): Due to liver disease, hypermetabolic states, and inhibition of pyruvate dehydrogenase
- Some patients may develop lactic acidosis from both mechanisms (e.g., liver failure and shock).
- Measuring pyruvate can assist in the differentiation of Type A and Type B lactic acidosis.
- A lactate-to-pyruvate ratio >25:1 supports the underlying mechanism of tissue hypoxia or Type A lactic acidosis.
- A normal lactate-to-pyruvate ratio (10:1) supports Type B lactic acidosis.
- Anaerobic metabolism is not an uncommon physiologic derangement seen in the perioperative arena; it can result when there is a mismatch of oxygen delivery and demand:
- Hypoxia/hypoxemia: Esophageal or mainstem intubation, hypoxic mixture, hypoventilation, V/Q mismatch, shunt
- Hypoperfusion: Hypotension, hypovolemia, hemorrhage, myocardial impairment, shock
- Vasodilation: Anesthetic medications, neuraxial blocks; anaphylactic or septic shock
- Cardiopulmonary bypass: Lactate is associated with malperfusion (2). It not only allows for prognostication, but also serves as an indicator of the efficacy of therapies used to restore tissue perfusion.
- Hyperlactatemia is associated with longer intensive care stays and increased postoperative ventilatory support, renal dysfunction, infectious complications, and circulatory disorders.
- Titrating therapies to traditional endpoints may not ensure that the microvascular bed is perfused. for example, a normal or high blood pressure may be a vasoconstrictive response to a low cardiac output state.
- Septic shock: Hyperlactatemia is typically present in patients with sepsis or septic shock and the etiology is multifactorial (3).
- Hypovolemia: Septic shock is associated with fluid-responsive physiology. Thus, an elevated lactate level could indicate a "dry" patient.
- Hypoperfusion: Sepsis is also accompanied by a hypermetabolic state with enhanced glycolysis. Patients with "normal" filling pressures (e.g., central venous pressure) and cardiac indices (e.g., cardiac index) may not have adequate oxygen delivery.
- Cytopathic hypoxia: Despite adequate volume status and perfusion, tissue dysfunction at the cellular level may persist in sepsis and represents impaired cellular function.
- Lactate is a well-established prognostic indicator in sepsis and septic shock. Obtaining serial serum lactate levels aids in identifying tissue hypoperfusion in patients who are not hypotensive but are at risk for septic shock; an elevated lactate (>4 mmol/L or 36 mg/dL) likely indicates inadequate oxygen delivery. Early goal-directed therapy should be considered in patients with sepsis and/or an elevated lactate level.
- As part of the Surviving Sepsis Guidelines, a resuscitation bundle for patients with sepsis includes, but it is not limited to:
 2 Lactate + 2 ATP + 2 H2O
2 Lactate + 2 ATP + 2 H2O