Description
- Hypertrophic cardiomyopathy (HCM) describes idiopathic, abnormal left ventricle hypertrophy (LVH) (specifically an asymmetric septum).
- The obstructive variant, commonly referred to as hypertrophic obstructive cardiomyopathy (HOCM) can impair LV ejection of blood to the systemic circulation.
Epidemiology
Prevalence
- In the US population: 1:500 people. It is the most common genetic cardiovascular disease
- Males > females
- Most commonly presents in the third decade of life, but it may present in persons of any age.
Etiology/Risk Factors
Genetic predisposition. HCM is an autosomal dominant trait that causes mutations in sarcomeric proteins. This results in structural abnormalities: A catenoid (curved surface with the property of net zero curvature at all points) configuration of the septum, which results in myocardial cell hypertrophy and disarray.
Physiology/Pathophysiology
- The hypertrophied myocardium exhibits
- Decreased lusitropy. Abnormalities of the cardiac microcirculation deplete energy stores essential for the sequestration of calcium during diastole, resulting in subendocardial ischemia. This causes persistent interaction of the contractile elements during diastole and increased diastolic stiffness.
- Increased ejection fraction (EF) that is commonly >80% despite diastolic dysfunction and decreased compliance.
- Increased myocardial oxygen demand. Similar to LVH and aortic stenosis (AS), there is greater muscle mass.
- Decreased myocardial oxygen delivery. Hypertrophied muscle creates more resistance to perfusion.
- Decreased wall tension. T = (R ×
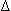 P)/H, where T = tension, R = radius,
P)/H, where T = tension, R = radius, 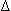 P = pressure gradient, and H = wall thickness
P = pressure gradient, and H = wall thickness - However, over time, the thickened heart may become weak and ineffective causing ventricular enlargement, systolic dysfunction (decreased EF), and dilated cardiomyopathy (increased wall tension).
- Abnormal sympathetic stimulation: Heightened responsiveness of the heart to the excessive production of catecholamines or the reduced neuronal uptake of norepinephrine might exacerbate HCM.
- Abnormally thickened intramural coronary arteries: These do not dilate normally, which leads to myocardial ischemia and, with time, can progress to myocardial wall fibrosis.
- Obstructive disease variant. Systolic anterior motion (SAM) of the anterior leaflet of the mitral valve can result in dynamic left ventricular outflow tract (LVOT) obstruction when the ventricle size decreases. Previously believed to result from high velocity flow and a Venturi effect on the anterior leaflet of the mitral valve, but recent echocardiographic evidence indicates that SAM is a low velocity phenomenon and that drag (the pushing force of flow), not the Venturi effect, is the dominant hydrodynamic force on the mitral leaflets. LVOT obstruction can be present at rest or can be induced with a Valsalva maneuver.
- Changes in electrical and muscular conduction and alterations of coronary perfusion increase the incidence of atrial and ventricular arrhythmia and risk for SCD.
- Maintenance of cardiac output, coronary perfusion, and prevention of cardiac decompensation are the primary goals.
- Monitor and maintain normal sinus rhythm.
- In patients with HOCM, decrease "emptying" of the heart by:
- Maintaining adequate intravascular volume
- Maintaining appropriate systemic vascular resistance (SVR): Avoid vasodilators, provide vasoconstriction
- Decreasing inotropy and chronotropy: Avoid inotropes and control sympathetic response with adequate anxiolytics and narcotics.