Description
- Pulmonary function tests (PFTs) measure lung volumes, airflow, and diffusing capacity of gas across the alveolar–capillary membrane (DLCO).
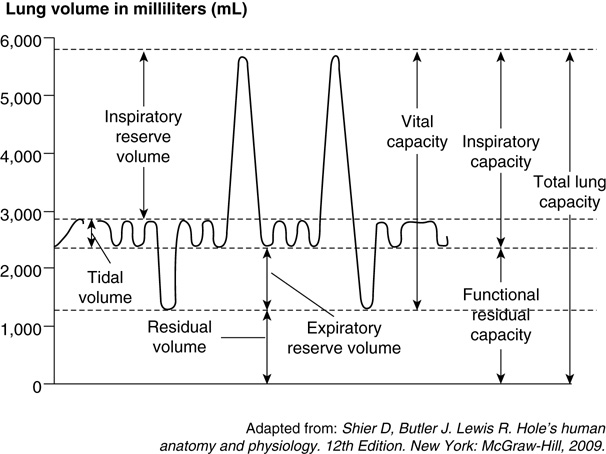
Lung volumes and capacities refer to the volume of air associated with different phases of the respiratory cycle.
FIGURE 1. Lung volumes and capacities.
- Lung volumes are directly measured. They include:
- Tidal volume (TV), inspiratory reserve volume (IRV), expiratory reserve volume (ERV), and residual volume (RV).
- Lung capacities are composed of one or more lung volumes. They include:
- Inspiratory reserve capacity (IRC), vital capacity (VC), total lung capacity (TLC), and functional residual capacity (FRC).
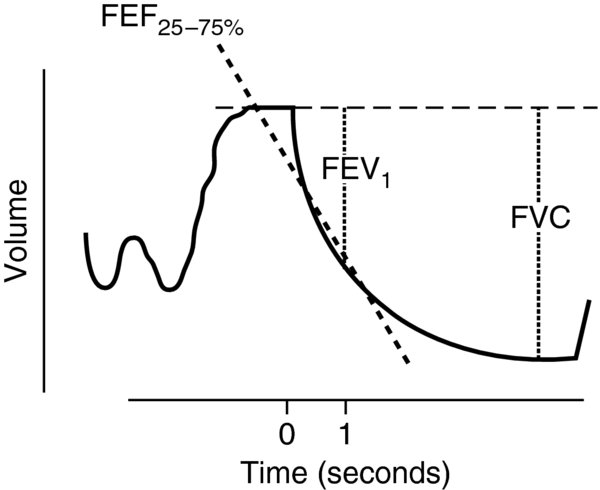
forced expiratory spirometry measures expiratory flow and volume while the patient forcefully exhales after maximal inhalation.
FIGURE 2. forced expiratory spirometry.
- TV: Volume of air inspired with passive breathing.
- Normal TV: ~6–8 mL/kg
- TV decreases with decreased lung compliance and/or reduced ventilatory muscle strength.
- IRV: Maximal volume of air that can be additionally inspired after a normal tidal inspiration.
- Normal IRV: ~1.9–3.3 L
- ERV: Maximum volume of air forcibly expired after a normal tidal expiration.
- Normal ERV: ~0.7–1 L
- RV: Volume of air remaining in the lungs after forceful expiration. It cannot be exhaled, and hence cannot be measured on routine spirometry.
- Normal RV: ~1 L
- Inspiratory capacity (IC): Volume of air inspired with maximum lung expansion after resting expiration.
- IC = IRV + TV (normal ~2.5 - 3.5 L)
- Reduced IC can signify extrathoracic airway obstruction.
- FRC: Volume of gas remaining in the lungs after passive expiration.
- FRC = ERV + RV (normal ~1.8 - 2.3 L)
- FRC influences the ventilation–perfusion relationship within the lung.
- Reduced FRC leads to an increase in venous admixture and arterial hypoxemia.
- Indirect determination of FRC (because RV cannot be measured on spirometry).
- Equilibration method: FRC is calculated by the equilibration of a tracer gas (helium) in a closed-circuit system. Using a spirometer and starting at FRC, the patient breathes a given concentration of helium and oxygen. Helium equilibrates between the patient's lungs and the spirometer. Since the system is closed, FRC can be determined by (Concentration1 × Volume1) = (Concentration2 × Volume2).
- Washout method: FRC is calculated from the washout of a tracer gas (nitrogen) within the lungs. The patient breathes 100% oxygen for several minutes to "wash out" alveolar nitrogen. Nitrogen fractional concentration and expired volume is measured over 7 minutes. The initial and final alveolar nitrogen fractional concentrations and the nitrogen concentration in the collected expirate are used to calculate FRC.
- Plethysmography: The patient sits in a box with a pressure transducer and breathes through a mouthpiece. The mouthpiece is connected to an electronic shutter and a differential pressure pneumotachometer. Mouth pressure and box pressure changes are measured during tidal breathing and panting maneuvers. The differential pressures are used to calculate thoracic gas volume and FRC.
- VC: The maximum volume of air that can be exhaled after maximal inspiration.
- VC = IRV + TV + ERV (normal ~60 mL/kg)
- VC correlates with the ability for deep breathing and effective coughing.
- TLC: The volume of air within the lungs after maximum inspiratory effort.
- TLC = IRV + TV + ERV + RV (normal ~ 5 L)
- forced vital capacity (FVC): The volume of air exhaled with maximal expiration after maximal inspiration.
- FVC normally equals VC.
- FVC measurements are dependent on patient effort and cooperation.
- forced expiratory volume in 1 second (FEV1): The volume of air exhaled forcefully in the first second of an FVC maneuver.
- Measure of flow (volume expired over time).
- Dependent on patient effort. Low FEV1 may be a result of inadequate effort, not lung disease.
- FEV1/FVC: Normalizes FEV1 measurements to the patient's individual lung volume.
- Normal FEV1/FVC
 0.75; the volume of air that is expired in 1 second should be 75% of the forced vital capacity.
0.75; the volume of air that is expired in 1 second should be 75% of the forced vital capacity.
- Normal FEV1/FVC
- forced midexpiratory flow rate (FEF25-75%): Exhaled volume between 25% and 75% of FVC.
- formerly called the maximum midexpiratory flow rate (MMFR).
- Less effort dependent than FEV1 or FVC, but may be reduced by submaximal inspiration (decreased FVC) or markedly decreased expiratory effort.
- Normal values vary widely due to the dependence on FVC. Both the absolute value (normal ~ 2–5 L/sec) and the percentage of predicted value (normal ~ 100% ± 25%) are recorded.
- Diffusing capacity (DLCO): Measures the diffusion of carbon monoxide (CO) across the alveolar–capillary membrane. Diffusion capacity is determined by measuring the partial pressure difference between inspired and expired CO.
- Maximum voluntary ventilation (MMV): Largest volume that can be breathed in 1 minute by voluntary effort.
- MMV records the patient breathing as rapidly and deeply as possible for 10 seconds by a pneumotachograph and the results are extrapolated to 1 minute.
- Best indicator of ventilatory muscle endurance.
Physiology/Pathophysiology
- Obstructive lung disease: Asthma, chronic bronchitis, emphysema, bronchiolitis, and chronic obstructive pulmonary disease (COPD).
- Diminished expiratory airflow from collapse of small airways.
- Decreased FEV1/FVC and FEF25-75%.
- Airway collapse during expiration causes air trapping with subsequent hyperinflation.
- Increased TLC, FRC, RV.
- Diminished expiratory airflow from collapse of small airways.
- Restrictive lung disease: Intrinsic and extrinsic pulmonary processes.
- Intrinsic (loss of alveolar airspace): Pulmonary fibrosis, sarcoidosis, or pneumonia.
- Extrinsic (reduction of lung space): Scoliosis, disorders of thoracic cage, pleural effusion, pneumothorax, or neuromuscular disorders.
- Smaller lung volumes along with normal or decreased expiratory flow (decreases to a lesser extent than volume).
- Normal or increased FEV1/FVC and FEF25-75%
- Reduction of all lung volumes, especially TLC, VC, and RV.
- Compromise of alveolar capillary unit: Loss of lung parenchyma (emphysema or COPD), thickening of the alveolar–capillary membrane (interstitial lung disease or pulmonary fibrosis), or pulmonary vascular disease.
- Reduction of DLCO
- Patients undergoing general anesthesia and surgical procedures (particularly thorax and upper abdomen) develop changes in pulmonary function that may contribute to postoperative pulmonary complications.
- VC is reduced up to 25–50% of preoperative values after open thorax or upper abdominal procedures and remains depressed for 10–14 days.
- FRC decreases by 10–15% in the supine position.
- The primary goal of preoperative PFTs is to recognize patients at high risk of postoperative pulmonary complications.
- Reduced FVC values (<15 mL/kg) are associated with postoperative pulmonary complications (1).
- Most commonly seen in quadriplegics or severe neuromuscular disease.
- Reduced FVC is associated with ineffective cough and development of atelectasis.
- Reduced FVC values (<15 mL/kg) are associated with postoperative pulmonary complications (1).
- PFTs provide some predictive benefit in patients undergoing lung resection.
- Predicted postoperative FEV1 calculated by multiplying the preoperative FEV1 by the percentage of lung tissue expected to remain after resection. An increased risk of perioperative pulmonary morbidity is associated with a predicted postoperative FEV1 <30% (2).
- IC = TV + IRV; where IC is inspiratory capacity, TV is tidal volume, and IRV is inspiratory reserve volume
- FRC = ERV + RV
- VC = IRV + TV + ERV; where VC is vital capacity, ERV is expiratory reserve volume, and IRV is inspiratory residual volume
- TLC = VC + RV; where TLC is total lung capacity, VC is vital capacity, and RV is residual volume