It is important to follow the most current Advanced Cardiac Life Support algorithms, which are updated regularly based on scientific evidence and best practices. In out-of-hospital resuscitations, the American Heart Association recommends chest-compression-only CPR, also known as cardiocerebral resuscitation. If the patient is in full arrest, use current CPR guidelines. CPR for patients in the hospital is performed with a compression-to-ventilation ratio of 30:2 until the defibrillator arrives. If the patient is not in full arrest, the first step of treatment is to maintain ABCs. Low-flow oxygen by nasal cannula or mask may decrease the rate of PVCs. Higher flow rates are usually needed for the patient with VT, and if pulseless VT or VF occurs, the patient needs immediate endotracheal intubation, and support of breathing with a manual resuscitator bag. The most important intervention for a patient with pulseless VT or VF is rapid defibrillation (electrical countershock). If a defibrillator is not available, and the arrest was witnessed, begin chest compressions and, as soon as possible, give a sharp blow to the precordium (precordial thump or thumpversion) to try to convert VT or VF into a regular sinus rhythm. Maintain CPR between all other interventions for patients without adequate breathing and circulation.
The drugs of choice to manage PVCs or VT with a pulse depend on the morphology of the ventricular beats and include amiodarone, lidocaine, or depending on the morphology of the electrocardiogram, magnesium sulfate. If the patient has pulseless VT or VF, the treatment of choice is to defibrillate the patient as discussed previously, intubate the patient, administer epinephrine, and then administer amiodarone or lidocaine. If the patient has electrolyte imbalances, or they are suspected, supplemental potassium, calcium, and/or magnesium is administered IV. Procainamide may be given to treat sustained ventricular tachycardia or recurrent VF if other interventions have not been successful. Long-term management may be done by ICDs.
The patient with ventricular asystole is managed with CPR. Initiate CPR, intubate the patient immediately, provide oxygenated breathing with a manual resuscitator bag, and obtain IV access. Confirm the ventricular asystole in a second lead to make sure the patient is not experiencing VF, which would indicate the need to defibrillate. If the rhythm still appears as ventricular asystole, administer epinephrine every 3 to 5 minutes in an attempt to have the patient regain an effective cardiac rhythm. Depending on setting and expertise, practitioners may consider a transcutaneous or transvenous pacemaker, but if efforts do not convert the cardiac rhythm, the physician may terminate resuscitation efforts.
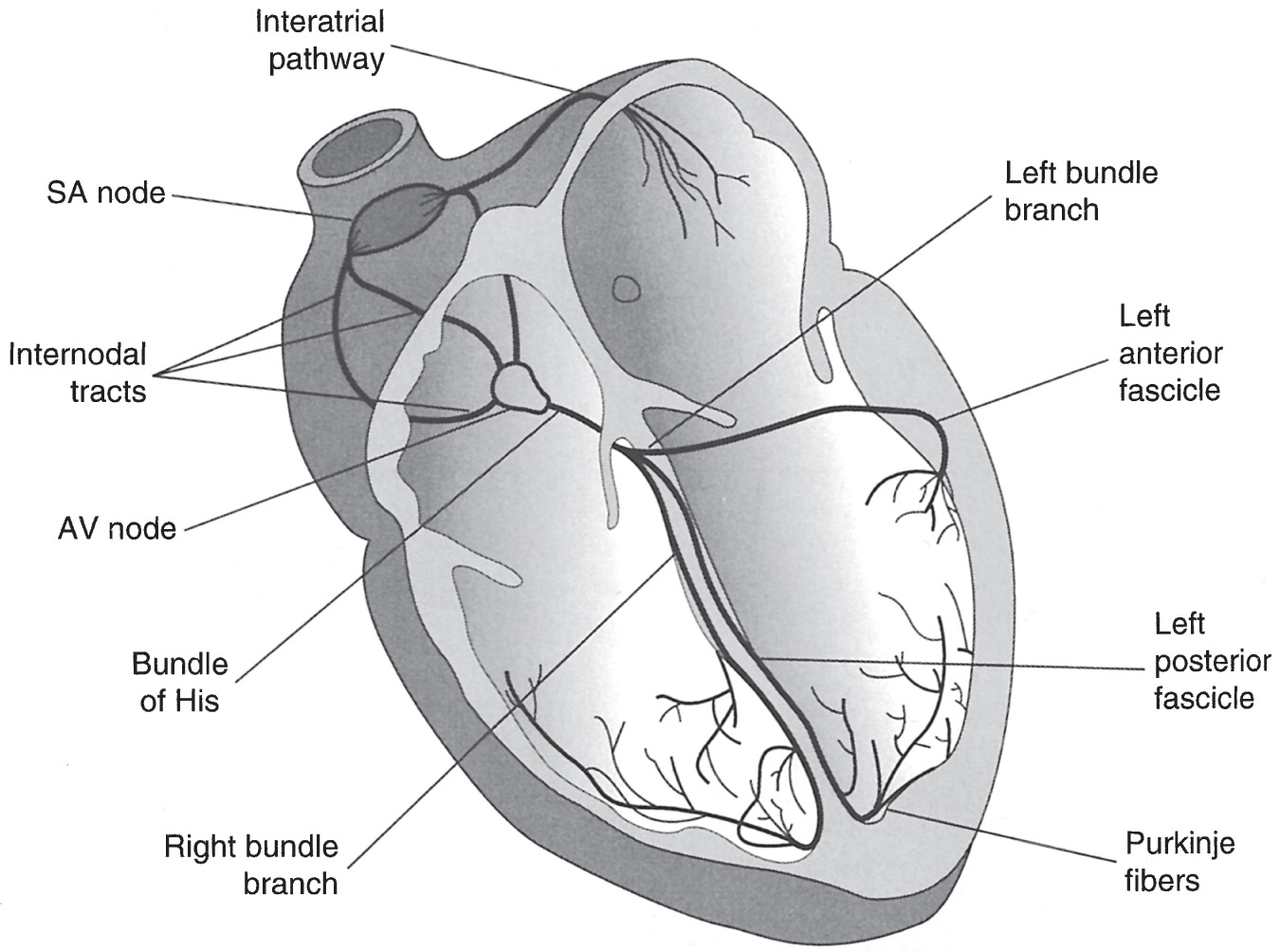 Figure 1 Electrical Conduction System of the Heart
Figure 1 Electrical Conduction System of the Heart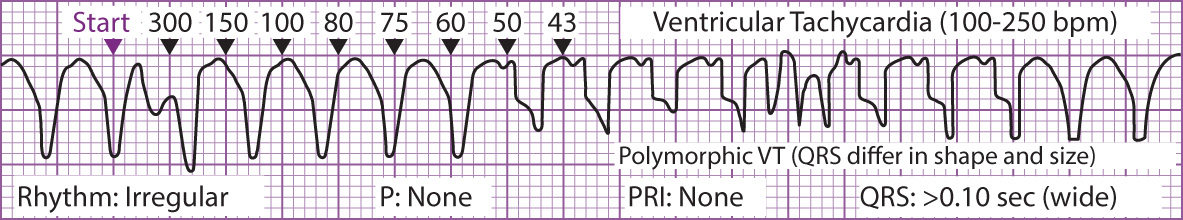 Figure 2 Ventricular Tachycardia
Figure 2 Ventricular Tachycardia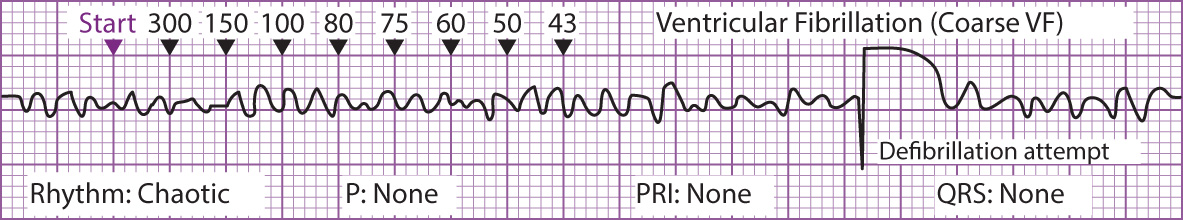 Figure 3 Ventricular Fibrillation
Figure 3 Ventricular Fibrillation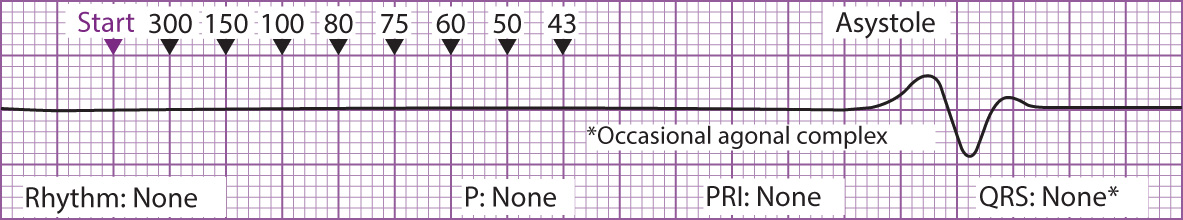 Figure 4 Asystole
Figure 4 Asystole