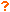Y-Site Injection Compatibility (1:1 Mixture)
- Acetaminophen:

- Albumin human:

- Amikacin sulfate:

- Aminophylline:

- Amiodarone HCl:

- Ampicillin sodium:

- Argatroban:

- Atracurium besylate:

- Bivalirudin:

- Butorphanol tartrate:

- Calcium chloride:

- Cangrelor tetrasodium:

- Cefazolin sodium:

- Cefepime HCl:

- Ceftazidime:

- Ceftolozane sulfate-tazobactam sodium:

- Chloramphenicol sodium succinate:

- Ciprofloxacin:

- Cisatracurium besylate:


- Clevidipine:
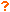
- Clindamycin phosphate:

- Cloxacillin sodium:

- Dexmedetomidine HCl:

- Diltiazem HCl:

- Dopamine HCl:

- Enalaprilat:

- Epinephrine:

- Eravacycline dihydrochloride:

- Erythromycin lactobionate:

- Esomeprazole sodium:

- Famotidine:

- Fenoldopam mesylate:

- Fentanyl citrate:


- Furosemide:


- Gentamicin sulfate:

- Heparin sodium:

- Hetastarch in lactated electrolyte:

- Hydrocortisone sodium succinate:


- Hydroxyethyl starch 130/0.4 in sodium chloride 0.9%:

- Ibuprofen:

- Imipenem-cilastatin sodium-relebactam:

- Insulin human:

- Isavuconazonium sulfate:

- Labetalol HCl:

- Levofloxacin:

- Levothyroxine sodium:

- Linezolid:

- Magnesium sulfate:

- Meropenem:


- Meropenem-vaborbactam:

- Metronidazole:

- Micafungin sodium:

- Midazolam HCl:

- Morphine sulfate:


- Nafcillin sodium:

- Nicardipine HCl:

- Nitroglycerin:

- Norepinephrine bitartrate:


- Pancuronium bromide:

- Pantoprazole sodium:

- Penicillin G potassium:

- Phenytoin sodium:

- Plazomicin sulfate:

- Polymyxin B sulfate:

- Potassium chloride:

- Potassium phosphates:

- Propofol:

- Remifentanil HCl:

- Remimazolam besylate:

- Sodium acetate:

- Sodium nitroprusside:

- Streptomycin sulfate:

- Sulbactam sodium and durlobactam sodium:

- Tacrolimus:

- Tedizolid phosphate:

- Tobramycin sulfate:

- Trimethoprim-sulfamethoxazole:

- Vancomycin HCl:

- Vecuronium bromide: