How to Use ASHP® Injectable Drug Information™
What Is ASHP® Injectable Drug Information™ ?
The ASHP® Injectable Drug Information™ is a collection of summaries of information from the published literature on the pharmaceutics of parenteral medications as applied to the clinical setting. ASHP® Injectable Drug Information™ is constructed from information derived from 4121 references with the information presented in the standardized structure described below. The purpose of this reference text is to facilitate the use of this clinical pharmaceutics research by knowledgeable health care professionals for the benefit of patients. The summary information from published research is supplemented with information from the labeling of each product and from other references.
The information base summarized in ASHP® Injectable Drug Information™ is large and highly complex, requiring thoughtful consideration for proper use. This reference text is not, nor should it be considered, elementary in nature or a primer. A single quick glance in a table is not adequate for proper interpretation of this highly complex information base. Proper interpretation includes the obvious need to consider and evaluate all relevant research information and results. Additionally, information on the formulation components (e.g., excipients), product attributes (especially pH), and the known stability behaviors of each parenteral drug, as well as the clinical situation of the patient, must be included in a thoughtful, reasoned evaluation of clinical pharmaceutics questions. Stability and sterility both must be considered in the assignment of beyond-use dates.
Who Should Use ASHP® Injectable Drug Information™?
ASHP® Injectable Drug Information™ is designed for use as a professional reference and guide to the literature on the clinical pharmaceutics of parenteral medications. The intended audience consists of knowledgeable healthcare professionals, particularly pharmacists, who are well versed in the formulation and clinical use of parenteral medications and who have the highly specialized knowledge base, training, and skill set necessary to interpret and apply the information. Practitioners who are not well versed in the formulation, essential properties, and clinical application of parenteral drugs should seek the assistance of more knowledgeable and experienced healthcare professionals to ensure patient safety.
Users of ASHP® Injectable Drug Information™ must recognize that no reference work, including this one, can substitute for adequate decision-making by healthcare professionals. Proper clinical decisions must be made after considering all aspects of the patient’s condition and needs, with particular attention to the special demands imposed by parenteral medications. ASHP® Injectable Drug Information™ cannot make decisions for its users. However, in knowledgeable hands, it is a valuable tool for the proper use of parenteral medications.
Organization of ASHP® Injectable Drug Information™
ASHP® Injectable Drug Information™ has been organized as a collection of monographs on each of the drugs. The monographs are arranged alphabetically by nonproprietary (generic) name. The nonproprietary names of the drugs are the United States Adopted Names (USAN) and other official names for drugs as described in the USP Dictionary of USAN and International Drug Names. Also included are some of the trade (proprietary, brand) names and manufacturers of the drug products; this listing is not necessarily comprehensive and should not be considered an endorsement of any product or manufacturer.
All of the information included in ASHP® Injectable Drug Information™ is referenced so that those who wish to study the original sources may find them. Efforts are ongoing to provide increased specificity of references to product labeling as individually cited references to the labeling for a drug product, including the proprietary name (if available), manufacturer, and revision date of the prescribing information; this will facilitate location of specific labeling that has been used as a reference. In addition, the full list of the AHFS® Pharmacologic-Therapeutic Classification© system and specific classification numbers within each individual monograph have been included to facilitate the location of therapeutic information on the drugs.
The monographs have been divided into the subheadings described below:
Products—lists many of the sizes, strengths, volumes, and forms in which the drug is supplied, along with other components of the formulation. Instructions for reconstitution (when applicable) are included in this section.
The products described do not necessarily comprise a comprehensive list of all available products. Rather, some common representative products are described. Furthermore, dosage forms, sizes, and container configurations of parenteral products may undergo important changes during the lifespan of this edition of ASHP® Injectable Drug Information™.
Following the product descriptions, the pH of the drug products, the osmotic value(s) of the drug and/or dilutions (when available), and other product information such as the sodium content and definition of units are presented.
Practitioners have not always recognized the value and importance of incorporating product formulation information into the thought process that leads to their decision on handling drug compatibility and stability questions. Excipients used in the formulation of commercially available products may vary among manufacturers and can influence drug compatibility and stability; specific product labeling should be consulted for additional formulation details. Consideration of the product information and formulation components, as well as the properties and attributes of the products (especially pH), is essential to proper interpretation of the information presented in ASHP® Injectable Drug Information™.
Administration—includes route(s) by which the drug can be given, rates of administration (when applicable), and other related administration details.
The administration information is a condensation derived principally from product labeling. For complete information, including dosage information sufficient for prescribing, the reader should refer to product labeling and therapeutically comprehensive references, such as the AHFS® Drug Information®.
Stability—describes the drug’s stability and storage requirements. The storage condition terminology of The United States Pharmacopeia, 42nd ed., is used in ASHP® Injectable Drug Information™.
The United States Pharmacopeia defines controlled room temperature as a temperature that encompasses the usual and customary working environment of 20-25°C and that results in a mean kinetic temperature no greater than 25°C.17 Temperature excursions between 15-30°C that are experienced in pharmacies, hospitals, warehouses, and during shipping are permitted.17 Transient temperature elevations up to 40°C also are permitted provided that they do not persist beyond 24 hours and that the mean kinetic temperature does not exceed 25°C.17 In contrast, room (ambient) temperature is defined simply as a temperature prevailing in a working environment.17
Protection from excessive heat is often required; excessive heat is defined as any temperature above 40°C.17 Similarly, protection from freezing may be required for products that are subject to loss of strength or potency or to destructive alteration of their characteristics, in addition to the risk of container breakage, upon exposure to freezing temperatures.17
Some products may require storage at a cool temperature, which is defined as any temperature between 8-15°C, or a cold temperature, which is defined as any temperature not exceeding 8°C.17 A refrigerator is defined as a cold place in which the temperature is controlled between 2-8°C.17 A freezer refers to a place in which the temperature is controlled between -25 and -10°C.17 Some products may have a recommended storage condition below -20°C, and in such cases, the temperature should be controlled to within 10°C of -20°C.17
In addition to storage requirements, aspects of drug stability related to pH, freezing, and exposure to light are presented in this section. Also presented is information on repackaging of the drugs or their dilutions in container/closure systems other than the original package (e.g., prefilling into syringes or in ambulatory pump reservoirs). Sorption and filtration characteristics of the drugs are provided as well when this information is available. The information is derived principally from the primary published research literature and is supplemented with information from other sources when available.
Standardize 4 Safety
The Stability section also contains information on Standardize 4 Safety where applicable. Standardize 4 Safety is a national patient safety initiative to standardize concentrations of drugs, including intravenous drugs, to reduce medication errors. An expert panel was convened to determine the recommended standard concentrations. Because recommendations from the Standardize 4 Safety panel may differ from the manufacturer’s prescribing information, caution is advised when using concentrations that differ from labeling, particularly when using rate information from the labeling. For additional information on Standardize 4 Safety, including updates that may be available, please see www.ashp.org/standardize4safety.
Compatibility Information—tabulates the compatibility results of the drug about which the monograph is written with infusion solutions and other drugs based on published reports from the primary research as well as the product labeling. The various entries are listed alphabetically by solution or drug name; the information is completely cross-referenced among the monographs.
Four types of tables are utilized to present the available information, depending on the kind of test being reported. The first type is for information on the compatibility of a drug in various infusion solutions and is depicted in Table 1. The second type of table presents information on two or more drugs in intravenous solutions and is shown in Table 2. The third type of table is used for tests of two or more drugs in syringes and is shown in Table 3. The fourth table format is used for reports of simulated or actual injection into Y-sites and manifolds of administration sets and is shown in Table 4.
Many published articles, especially older ones, do not include all of the information necessary to complete the tables. However, the tables have been completed as fully as possible from the original articles; in some cases, editorial staff have supplemented the published information based on direct communication with the authors of the published research.
Table 1. Solution Compatibility
| Monograph drug name | ||||||
|---|---|---|---|---|---|---|
| Solution | Mfr | Mfr | Conc/L or % | Remarks | Ref | C/I |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) |
| ||||||
Table 2. Additive Compatibility
| Monograph drug name | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Mfr | Conc/L or % | Mfr | Conc/L or % | Test Soln | Remarks | Refs | C/I |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) |
| ||||||||
Table 3. Drugs in Syringe Compatibility
| Monograph drug name | |||||||
|---|---|---|---|---|---|---|---|
| Drug (in syringe) | Mfr | Amt | Mfr | Amt | Remarks | Ref | C/I |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) |
| |||||||
Table 4. Y-Site Injection Compatibility (1:1 Mixture)
| Monograph drug name | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Mfr | Conc | Mfr | Conc | Remarks | Ref | C/I |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) |
| |||||||
Additional Compatibility Information—provides additional information and discussions of compatibility presented largely in narrative form.
Other Information—contains any relevant auxiliary information concerning the drug that does not fall into the previous categories.
The concentrations of all admixtures in intravenous solutions in the tables (Table 1 and Table 2) generally have been indicated in terms of concentration per liter (L) to facilitate comparison of the various studies. In some cases, this may result in amounts of the drug that are greater or lesser than those normally administered (as when the recommended dose is tested in 100 mL of vehicle), but the listings do accurately reflect the actual concentrations tested, expressed in standardized terms. Concentration may be expressed as a percentage for certain drugs or solutions (e.g., dextran, hetastarch, mannitol, lipid emulsion) to reflect the style commonly used to express concentration for these products.
For studies involving syringes, the actual amounts used are indicated. The volumes are also listed if available.
For studies of actual or simulated Y-site injection of drugs, the concentrations are cited in terms of concentration per mL of each drug solution prior to mixing at the Y-site. Most published research reports have presented the drug concentrations in this manner, and ASHP® Injectable Drug Information™ follows this convention. For those few published reports that presented the drug concentrations after mixing at the Y-site, the concentrations have been recalculated to be consistent with the more common presentation style to maintain the consistency of presentation in ASHP® Injectable Drug Information™. Note that the Y-Site Injection Compatibility table is designed with the assumption of a 1:1 mixture of the subject drug and infusion solution or admixture. For citations reporting other than a 1:1 mixture, the actual amounts tested are specifically noted.
Designating Compatibility or Incompatibility
Each summary of a published research report appearing in the Compatibility Information tables bears a compatibility indicator (C, I, or ?). A report receives a designation of C when the study results indicate that compatibility of the test samples existed under the test conditions. If the study determined an incompatibility existed under the test conditions, then an I designation is assigned for the ASHP® Injectable Drug Information™ entry for that study result. A designation of ? indicates that the test result does not clearly fit either the compatibility or incompatibility definition. Specific standardized guidelines are used to assign these compatibility designations and are described below. The citation is designated as a report of compatibility when results of the original article indicated one or more of the following criteria were met:
- Physical or visual compatibility of the combination was reported (no visible or electronically detected indication of particulate formation, haze, precipitation, color change, or gas evolution).
- Stability of the components for at least 24 hours in an admixture under the specified conditions was reported (decomposition of 10% or less).
- Stability of the components for the entire test period, although in some cases it was less than 24 hours, was reported (time periods less than 24 hours have been noted).
The citation is designated as a report of incompatibility when the results of the original article indicated either or both of the following criteria were met:
- A physical or visual incompatibility was reported (visible or electronically detected particulate formation, haze, precipitation, color change, or gas evolution).
- Greater than 10% decomposition of one or more components in 24 hours or less under the specified conditions was reported (time periods less than 24 hours have been noted).
Reports of test results that do not clearly fit into the compatibility or incompatibility definitions cannot be designated as either. These are indicated with a question mark.
Although these criteria have become the conventional definitions of compatibility and incompatibility, the reader should recognize that the criteria may need to be tempered with professional judgment. Inflexible adherence to the compatibility designations should be avoided. Instead, they should be used as aids in the exercising of professional judgment.
Therapeutic incompatibilities or other drug interactions are not within the scope of ASHP® Injectable Drug Information™ and are therefore not addressed. Therapeutically comprehensive references and the product labeling should be consulted for such information.
Interpreting Compatibility Information in ASHP® Injectable Drug Information™
As mentioned above, the body of information summarized in ASHP® Injectable Drug Information™ is large and complicated. With the possible exception of a report of immediate gross precipitation, it usually takes some degree of thoughtful consideration and judgment to properly evaluate and appropriately act on the research results that are summarized in this resource.
Nowhere is the need for judgment more obvious than when apparently contradictory information appears in two or more published reports. The body of literature in drug-drug and drug-solution compatibility is replete with apparently contradictory results. Except for study results that have been documented later to be incorrect, the conflicting information has been included in ASHP® Injectable Drug Information™ to provide practitioners with all of the information for their consideration. The conflicting information will be readily apparent to the reader because of the content of the Remarks section as well as the C, I, and ? designations following each citation.
Many or most of the apparently conflicting citations may be the result of differing conditions or materials used in the studies. A variety of factors that can influence the compatibility and stability of drugs must be considered in evaluating such conflicting results, and absolute statements are often difficult or impossible to make. Differences in concentrations, buffering systems, preservatives, vehicles, temperatures, and order of mixing all may play a role. By reviewing a variety of reports, the user of ASHP® Injectable Drug Information™ is better able to exercise professional judgment with regard to compatibility and stability.
The reader must guard against misinterpretation of research results, which may lead to inappropriate assumptions of compatibility and stability. As an example, a finding of precipitate formation two hours after two drugs are mixed does not imply nor should it be interpreted to mean that the combination is compatible until that time point, when a sudden precipitation occurs. Rather, it should be interpreted to mean that precipitation occurred at some point between mixing and the first observation point at two hours. Such a result would lead to a designation of incompatibility in ASHP® Injectable Drug Information™.
Precipitation reports can be particularly troublesome for practitioners to deal with because of the variability of the time frames in which they may occur. Apart from combinations that repeatedly result in immediate precipitation, the formation of a precipitate can be unpredictable to some degree. Numerous examples of variable precipitation time frames can be found in the literature, including paclitaxel, etoposide, and sulfamethoxazole-trimethoprim (co-trimoxazole) in infusion solutions and calcium and phosphates precipitation in parenteral nutrition mixtures (e.g., TNAs, TPNs). Differing drug concentrations also can play a role in creating variability in results. A good example of this occurs with co-administered vancomycin hydrochloride and beta-lactam antibiotics. Users of the information in ASHP® Injectable Drug Information™ must always be aware that a marginally incompatible combination might exhibit precipitation earlier or later than that reported in the literature. In many such cases, the precipitation is ultimately going to occur, it is just the timing that is in question. This is of particular importance for precipitate formation because of the potential for serious adverse clinical consequences, including death, that have occurred. Certainly, users of ASHP® Injectable Drug Information™ information should always keep in mind and anticipate the possibility of precipitation and its clinical ramifications. Furthermore, all injections and infusions should be inspected for particulate matter and discoloration prior to administration. If found, such injections and solutions should be discarded.
In addition, many research reports cite test solutions or concentrations that may not be appropriate for clinical use. An example would be a report of a drug’s stability in unsterile water. Although the ASHP® Injectable Drug Information™ summary will accurately reflect the test solutions and conditions that existed in a study, it is certainly inappropriate to misinterpret a stability report like this as being an authorization to use the product clinically. In such cases, the researchers may have used the clinically inappropriate diluent to evaluate the drug’s stability for extrapolation to a more suitable vehicle that is similar, or they may not have recognized that the diluent is clinically unsuitable. In either event, it is incumbent on the practitioner in the clinical setting to use professional judgment to apply the information in an appropriate manner and recognize what is not acceptable clinically.
Further, it should be noted that many of the citations designated incompatible are not absolute. While a particular admixture may incur more than 10% decomposition within 24 hours, the combination may be useful for a shorter time period. The concept of “utility time” or the time to 10% decomposition may be useful in these cases. Unfortunately, such information is often not available. Included in the Remarks columns of the tables are the amount of decomposition, the time period involved, and the temperature at which the study was conducted when this information is available.
Users of ASHP® Injectable Drug Information™ information should always keep in mind that the information contained in this reference text must be used as a tool and a guide to the research that has been conducted and published. It is not a replacement for thoughtfully considered professional judgment. It falls to the practitioner to interpret the information in light of the clinical situation, including the patient’s needs and status. What is certain is that relying solely on the C or I designation without the application of professional judgment is inappropriate.
In addition to conflicting information, many of the published articles have provided only partial evaluations, not looking at all aspects of a drug’s stability and compatibility. This is not surprising considering the complexity, difficulty, and costs of conducting such research. There are, in fact, articles that do provide evaluations of both physical stability/compatibility and chemical stability. But some are devoted only to physical issues, while others examine only chemical stability. Although a finding of precipitation, haze, or other physical effects may constitute an incompatibility (unless transient), the lack of such changes does not rule out chemical deterioration. In some cases, drugs initially designated as compatible because of a lack of visual change were later shown to undergo chemical decomposition. Similarly, the determination of chemical stability does not rule out the presence of unacceptable levels of particulates and/or turbidity in the combination. In a classic case, the drugs leucovorin calcium and fluorouracil were determined to be chemically stable for extended periods by stability-indicating HPLC assays in several studies, but years later, repeated episodes of filter clogging led to the discovery of unacceptable quantities of particulates in combinations of these drugs. The reader must always bear in mind these possibilities when only partial information is available.
And, finally, contemporary practitioners have come to expect that the analytical methods used in reports on the chemical stability of drugs will be validated, stability-indicating methods. However, many early studies used methods that were not demonstrated to be stability indicating.
Biological drugs (therapeutic proteins [e.g., enzymes, monoclonal antibodies, immune globulins]) are particularly sensitive to environmental factors and undergo more complex and numerous degradation pathways than classical drugs.3207; 3208; 3212 In addition to physicochemical instability issues similar to those observed with classical drugs (e.g., precipitation, decomposition), such proteins are subject to other stability issues (e.g., protein conformation, biologic activity) that must be considered.3207; 3208; 3209; 3212 Therefore, a single analytic method that only assesses protein concentration is insufficient to determine stability of biological products.3209; 3210 Interpretation of the results of compatibility and stability studies of such proteins poses a challenge because both analytic methods and meaningful acceptance criteria should be specific to the biologic; official compendial standards (e.g., The United States Pharmacopeia monographs and analytic methods) should be consulted when available.17; 3209; 3212 Although many experts agree that multiple complementary methods should be used to assess the physical and chemical stability as well as assessment of biologic activity, no clear guideline or recommendation for in-use stability studies for therapeutic proteins is available.3208; 3210; 3211; 3212
Literature Search for Updating ASHP® Injectable Drug Information™
To gather the bulk of the published compatibility and stability information for updating ASHP® Injectable Drug Information™, a literature search is performed using PubMed. By using key terms (e.g., stability), a listing of candidate articles for inclusion in ASHP® Injectable Drug Information™ is generated. From this list, relevant articles are critically evaluated and prioritized for inclusion. As a supplement to this automated literature searching, a manual search of the references of the articles is also conducted, and any articles not included previously are similarly evaluated for inclusion. In addition, pharmaceutical manufacturers may be contacted for additional in-house (unpublished) data.
| AA | Amino acids (percentage specified) |
| D | Dextrose solution (percentage unspecified) |
| D5LR | Dextrose 5% in Ringer’s injection, lactated |
| D5R | Dextrose 5% in Ringer’s injection |
| D-S | Dextrose-saline combinations |
| D2.5½S | Dextrose 2.5% in sodium chloride 0.45% |
| D2.5S | Dextrose 2.5% in sodium chloride 0.9% |
| D5¼S | Dextrose 5% in sodium chloride 0.225% |
| D5½S | Dextrose 5% in sodium chloride 0.45% |
| D5S | Dextrose 5% in sodium chloride 0.9% |
| D10S | Dextrose 10% in sodium chloride 0.9% |
| D5W | Dextrose 5% |
| D10W | Dextrose 10% |
| IM | Isolyte M |
| IP | Isolyte P |
| IS10 | Invert sugar 10% |
| I.U.a | International unit(s) |
| LR | Ringer’s injection, lactated |
| NM | Normosol M |
| NR | Normosol R |
| NRD5W | Normosol R in dextrose 5% |
| NS | Sodium chloride 0.9% |
| R | Ringer’s injections |
| REF | Refrigeration |
| RT | Room temperature |
| S | Saline solution (percentage unspecified) |
| ½S | Sodium chloride 0.45% |
| SL | Sodium lactate 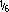 M M |
| TNA | Total nutrient admixture (3-in-1) |
| TPN | Total parenteral nutrition (2-in-1) |
| W | Sterile water for injection |
aIt is well accepted that “IU” is not a preferred abbreviation for international units. There is some difference of opinion among medication safety stakeholders on whether such units should be referred to simply as “units” or as “International Units.” Throughout the text of this reference, we generally have used the term international units to describe such units; however, because of space limitations within the columns of the Compatibility Information tables, the term international units has been abbreviated as I.U. where necessary.
Manufacturer and Compendium Abbreviations
| AB | Abbott |
| ABV | AbbVie |
| ABX | Abraxis |
| ACA | Acacia |
| ACC | American Critical Care |
| ACD | Accord Healthcare |
| ACH | Achaogen |
| ACR | Acrotech Biopharma |
| ACT | Actavis |
| AD | Adria |
| AFT | AFT |
| AGT | Aguettant |
| AH | Allen & Hanburys |
| AHP | Ascot Hospital Pharmaceuticals |
| AKN | Akorn |
| ALL | Allergan |
| ALP | Alpharma |
| ALS | Alfasigma |
| ALT | Altana Pharma |
| ALV | Alveda Pharma |
| ALX | Alexion Pharmaceuticals |
| ALZ | Alza |
| AM | ASTA Medica |
| AMA | AMAG |
| AMB | Amneal |
| AMD | Amdipharm |
| AMG | Amgen |
| AMP | Amphastar |
| AMR | American Regent |
| AMS | Amerisource |
| AND | Andromaco |
| ANP | Anpharm |
| ANT | Antigen |
| AP | Asta-Pharma |
| APC | Apothecon |
| APN | Aspen |
| APO | Apotex |
| APP | American Pharmaceutical Partners |
| APR | Aspri |
| APT | Aspen Triton |
| AQ | American Quinine |
| AR | Armour |
| ARC | American Red Cross |
| ARD | Ardeapharm |
| ARR | Arrow |
| ARV | Aurovitas |
| AS | Arnar-Stone |
| ASC | Ascot |
| ASK | Aska |
| ASP | Astellas Pharma |
| AST | Astra |
| ASZ | AstraZeneca |
| AT | Alpha Therapeutic |
| ATX | Athenex |
| AUB | Aurobindo |
| AUR | Auromedics |
| AVD | Avadel Legacy Pharmaceuticals |
| AVE | Aventis |
| AVT | Avet |
| AVG | Alvogen |
| AW | Asta Werke |
| AY | Ayerst |
| AZV | Laboratórios Azevedos |
| BA | Baxter |
| BB | B & B Pharmaceuticals |
| BAN | Banyu Pharmaceuticals |
| BAY | Bayer |
| BC | Bencard |
| BCT | BioCryst Pharmaceuticals |
| BD | Becton Dickinson |
| BE | Beecham |
| BED | Bedford |
| BEH | Behring |
| BEL | R. Bellon |
| BFM | Bieffe Medital |
| BI | Boehringer Ingelheim |
| BIO | Bioniche Pharma |
| BK | Berk |
| BKN | Baker Norton |
| BM | Boehringer Mannheim |
| BMS | Bristol-Myers Squibb |
| BN | Breon |
| BP | British Pharmacopoeiaa |
| BPC | British Pharmaceutical Codexa |
| BPI | BPI Labs |
| BR | Bristol |
| BRD | Bracco Diagnostics |
| BRK | Breckenridge |
| BRN | B. Braun |
| BRT | Britianna |
| BT | Boots |
| BTG | BTG International |
| BTK | Biotika |
| BV | Ben Venue |
| BW | Burroughs Wellcome |
| BX | Berlex |
| BXT | Baxalta |
| CA | Calmic |
| CAD | Cadence Pharmaceuticals |
| CAN | Cangene Biopharma |
| CAR | Cardinal Health |
| CBH | CSL Behring |
| CDL | Cadila |
| CDM | CDM Lavoisier |
| CE | Carlo Erba |
| CEL | Celgene |
| CEN | Centocor |
| CER | Cerenex |
| CET | Cetus |
| CH | Labo Choay |
| CHE | Chephasaar Chemisch-Pharmazeutische |
| CHI | Chiron |
| CHP | Cheplapharm |
| CHS | Chiesi |
| CHU | Chugai |
| CI | Ciba |
| CIP | Cipla |
| CIS | CIS US |
| CL | Clintec |
| CLA | Claris Lifesciences |
| CLN | Clinigen |
| CMB | Cumberland |
| CMP | CMP Pharma |
| CN | Connaught |
| CNF | Centrafarm |
| CO | Cole |
| COM | CommScope |
| COR | COR Therapeutics |
| COV | Covis |
| CP | Continental Pharma |
| CPP | CP Pharmaceuticals |
| CPR | Cooper |
| CR | Critikon |
| CRC | Caraco |
| CRP | Carinopharm GmbH |
| CSL | CSL Ltd. |
| CTI | Cell Therapeutics Inc. |
| CU | Cutter |
| CUB | Cubist |
| CUP | Cura Pharmaceuticals |
| CUR | Curomed |
| CY | Cyanamid |
| DAK | Dakota |
| DB | David Bull Laboratories |
| DCC | Dupont Critical Care |
| DFK | Dr. Franz Köhler Chemie |
| DGL | Douglas |
| DI | Dista |
| DIA | Diamant |
| DM | Dome |
| DME | Dupont Merck Pharma |
| DMX | Dumex |
| DRA | Dr. Rentschler Arzneimittel |
| DRT | Durata Therapeutics |
| DRX | Draxis |
| DSC | Daiichi Sankyo |
| DU | DuPont |
| DUR | Dura |
| DW | Delta West |
| EA | Eaton |
| EBE | Ebewe |
| ECL | Éclat |
| EGL | Eagle |
| EI | Eisai |
| ELN | Elan |
| EMD | EMD Serono |
| EN | Endo |
| ENH | Enhua |
| ENT | Entasis |
| ENZ | Enzon |
| ERF | Erfa |
| ERM | Erempharma |
| ES | Elkins-Sinn |
| ESL | ESI Lederle |
| ESP | ESP Pharma |
| EST | Esteve |
| ETC | Etercon |
| ETH | Ethica |
| EV | Evans |
| EX | Essex |
| EXL | Exela |
| FA | Farmitalia |
| FAC | Facta Farmaceutical |
| FAN | Fandre Laboratories |
| FAU | Faulding |
| FC | Frosst & Cie |
| FED | Federa |
| FER | Ferring |
| FI | Fisons |
| FIN | Finusolprima |
| FOR | Forest Laboratories |
| FP | Faro Pharma |
| FRE | Fresenius |
| FRK | Fresenius Kabi |
| FUA | Fuan Pharmaceutical Group |
| FUJ | Fujisawa |
| GEI | Geistich Pharma |
| GEM | Geneva-Marsam |
| GEN | Genentech |
| GER | Gerda |
| GG | Geigy |
| GH | Generic Health |
| GIL | Gilead |
| GIU | Giulini |
| GL | Glaxo |
| GLA | Gland |
| GLT | Generics Limited |
| GMP | Generic Medical Partners |
| GNF | Genfarma |
| GNS | Gensia-Sicor |
| GO | Goedecke |
| GRI | Grifols |
| GRO | Grossen Apotheke |
| GRP | Gruppo |
| GRU | Grunenthal |
| GSK | GlaxoSmithKline |
| GVA | Geneva |
| GW | Glaxo Wellcome |
| GZ | Genzyme |
| HAE | Haemonetics |
| HAM | HameIn |
| HB | Hoechst-Biotika |
| HC | Hillcross |
| HE | Hengrui Medicine Co. |
| HEL | Helsinn |
| HER | Heritage |
| HIK | Hikma |
| HMR | Hoechst Marion Roussel |
| HO | Hoechst-Roussel |
| HOP | Hope Pharmaceuticals |
| HOS | Hospira |
| HQS | HQ Specialty Pharma |
| HR | Horner |
| HRN | Heron |
| HY | Hyland |
| ICI | ICI Pharmaceuticals |
| ICN | ICN Pharmaceuticals |
| ICU | ICU Meds |
| IMM | Immunex |
| IMS | IMS Ltd. |
| IN | Intra |
| INF | Infectopharm |
| INR | Inresa GmbH |
| INT | Intermune |
| IV | Ives |
| IVX | Ivex |
| IX | Invenex |
| JAZ | Jazz |
| JC | Janssen-Cilag |
| JHP | JHP Pharmaceuticals |
| JJ | Johnson & Johnson |
| JN | Janssen |
| JP | Jones Pharma |
| JUN | Juno Pharmaceuticals |
| KA | Kabi |
| KAL | Kalbe |
| KE | Kern Pharma |
| KED | Kedrion |
| KEY | Key Pharmaceuticals |
| KLI | Klinge Pharma |
| KLN | Kelun |
| KN | Knoll |
| KP | Kabi Pharmacia |
| KV | Kabi-Vitrum |
| KY | Kyowa |
| LA | Lagap |
| LE | Lederle |
| LED | Leadiant |
| LEI | Leiras |
| LEK | Lek |
| LEM | Lemmon |
| LEO | Leo Laboratories |
| LFB | Laboratoire Français du Fractionnement et des Biotechnologies |
| LI | Lilly |
| LIF | Lifeshield |
| LJ | La Jolla |
| LME | Laboratoire Meram |
| LNK | Link |
| LUN | Lundbeck |
| LXO | Laboratoire XO |
| LY | Lyphomed |
| LZ | Labaz Laboratories |
| MA | Mallinckrodt |
| MAC | Maco Pharma |
| MAR | Marsam |
| MAY | Mayne Pharma |
| MB | May & Baker |
| MCD | Merck Chibret Dohme |
| MDC | Medac |
| MDI | Medimmune |
| MDX | Medex |
| MDZ | Medicianz |
| ME | Merck & Co. |
| MEI | Meitheal Pharmaceuticals |
| MEL | Melinta Therapeutics |
| MER | Merus Labs |
| MG | McGaw |
| MGI | MGI Pharma |
| MI | Miles |
| MIC | Micro Labs |
| MIL | Millimed |
| MIP | MIP Pharma GmbH |
| MJ | Mead Johnson |
| MKG | Merck KGaA |
| MM | Merrimack |
| MMD | Marion Merrell Dow |
| MMS | Marian Merrell S.A. |
| MMT | Meridian Medical Technologies |
| MN | McNeil |
| MOC | Mochida |
| MON | Monarch |
| MPC | Maruishi Pharmaceutical |
| MPI | Mercury Pharma International |
| MRD | Merrell-Dow |
| MRN | Merrell-National |
| MRS | Mersi |
| MSD | Merck Sharp & Dohme |
| MTN | Marathon |
| MUN | Mundi Pharma |
| MY | Maney |
| MYL | Mylan |
| MYR | Mayrhofer Pharmazeutika |
| NA | National |
| NAB | Nabi |
| NAP | NAPP Pharmaceuticals |
| NCI | National Cancer Institute |
| NE | Norwich-Eaton |
| NEO | Neon Healthcare |
| NIN | Ningbo Team |
| NF | National Formularya |
| NO | Nordic |
| NOP | Novopharm |
| NOR | Normon |
| NOV | Novo Pharm |
| NVA | Novartis |
| NVL | Novell |
| NVN | Novo Nordisk |
| NVP | Nova Plus |
| NVX | Novex Pharma |
| NYC | Nycomed |
| OCT | Octapharma |
| ODH | Orpha-Devel Handels und Vertriebs |
| OHM | Ohmeda |
| OM | Omega |
| OMJ | OMJ Pharmaceuticals |
| OMN | Ortho-McNeil |
| ON | Orion |
| OR | Organon |
| ORC | Orchid |
| ORP | Orphan Medical |
| ORT | Ortho |
| OTS | Otsuka |
| OVA | Ovation |
| PAD | Paddock |
| PAL | Paladin |
| PAN | Panpharma Laboratory |
| PAR | Par |
| PB | Pohl-Boskamp |
| PCO | Pharmaco |
| PD | Parke-Davis |
| PE | Pentagone |
| PER | Perrigo |
| PF | Pfizer |
| PFM | Pfrimmer |
| PH | Pharmacia |
| PHA | Phapros |
| PHB | Phebra |
| PHC | Pharmachemie |
| PHM | Pharmacosmos |
| PHS | Pharmascience |
| PHT | Pharma-Tek |
| PHU | Pharmacia & Upjohn |
| PHX | Phoenix |
| PIR | Piramal |
| PMD | Promedica |
| PNN | Pantheon |
| PNT | Parenta |
| PO | Poulenc |
| PP | Pharmaceutical Partners |
| PPC | Pharmaceutical Partners of Canada |
| PPR | Premier Pro Rx |
| PR | Pasadena Research |
| PRF | Pierre Fabre |
| PRK | Parkfields |
| PRM | Premier |
| PRP | Premier Pro |
| PRS | Prasco Laboratories |
| PTK | Paratek |
| PTR | Partner Therapeutics |
| PX | Pharmax |
| QI | Qilu |
| QLM | Qualimed Labs |
| QU | Quad |
| RB | Robins |
| RBP | Ribosepharm |
| RC | Roche |
| REC | Recordati Pharma |
| REG | Regeneron |
| REN | Renaudin |
| RI | Riker |
| RKB | Reckitt Benckiser |
| RKC | Reckitt & Colman |
| ROR | Rorer |
| ROX | Roxane |
| RP | Rhone-Poulenc |
| RPR | Rhone-Poulenc Rorer |
| RR | Roerig |
| RS | Roussel |
| RTM | Rotexmedica GmbH |
| RTP | Ratiopharm |
| RU | Rugby |
| SA | Sankyo |
| SAA | Sanofi Aventis |
| SAG | Sageant |
| SAN | Sanofi |
| SB | Sintetica Bioren |
| SC | Schering |
| SCI | Scios |
| SCN | Schein |
| SCS | SCS Pharmaceuticals |
| SE | Searle |
| SEQ | Sequus |
| SER | Servier |
| SGA | SIGA Technologies |
| SGS | SangStat |
| SGT | Sagent |
| SGW | Serag-Wiessner GmbH & Co. KG |
| SHI | Shionogi |
| SHR | Shire |
| SIA | Siam Pharmaceutical |
| SIC | Sicor |
| SIG | Sigma Tau |
| SIN | Sintetica |
| SKB | SmithKline Beecham |
| SKF | Smith Kline & French |
| SLT | Slate Run |
| SM | Smith |
| SMX | SteriMax |
| SN | Smith + Nephew |
| SO | SoloPak |
| SP | Spectrum Pharmaceuticals |
| SPA | Steri-Pharma |
| SQ | Squibb |
| SQI | Seqirus |
| SRB | Serb |
| SRP | Sinopharm Rongsheng Pharmaceutical |
| SS | Sanofi-Synthelabo |
| ST | Sterilab |
| STP | Sterop |
| STR | Sterling |
| STS | Steris |
| STU | Stuart |
| SUN | Sun |
| SV | Savage |
| SW | Sanofi Winthrop |
| SX | Sabex |
| SXH | Shanghai Xudong Haipu |
| SY | Syntex |
| SYN | Synergen |
| SYO | Synthelabo |
| SZ | Sandoz |
| TAK | Takeda |
| TAL | Talon Therapeutics |
| TAP | TAP Holdings |
| TAR | Targanta Therapeutics |
| TAY | Taylor |
| TE | Teva |
| TEC | Teclapharm |
| TEL | Teligent |
| TER | Terumo |
| TES | Tesaro |
| TET | Tetraphase |
| TIL | Tillomed |
| TL | Tillotts |
| TMC | The Medicines Company |
| TO | Torigian |
| TOW | Towa |
| TR | Travenol |
| TRE | Trevena |
| UCB | UCB |
| UP | Upjohn |
| USB | US Bioscience |
| USP | United States Pharmacopeiaa |
| USV | USV Pharmaceuticals |
| UT | United Therapeutics |
| VAL | Valeant |
| VHA | VHA Plus |
| VI | Vitarine |
| VIA | Viatris |
| VIC | Vicuron Pharmaceuticals |
| VT | Vitrum |
| WAS | Wasserman |
| WAT | Watson |
| WAY | Wyeth-Ayerst |
| WB | Winthrop-Breon |
| WC | Warner-Chilcott |
| WDW | Woodward |
| WED | Weddel |
| WEL | Wellcome |
| WI | Winthrop |
| WG | WG Critical Care |
| WL | Warner Lambert |
| WOC | Wockhardt |
| WW | Westward |
| WY | Wyeth |
| XE | Xellia |
| XGN | XGen |
| XI | Xion Pharmaceuticals |
| XU | Xudong Pharmaceutical Co. |
| YAM | Yamanouchi |
| ZEN | Zeneca |
| ZLB | ZLB Biopharma |
| ZNS | Zeneus Pharma |
| ZY | ZyGenerics |
| ZYD | Zydus |
aWhile reference to a compendium does not indicate the specific manufacturer of a product, it does help to indicate the formulation that was used in the test.
ASHP® Injectable Drug InformationTM. Selected Revisions January 10, 2024. © Copyright, 2025. American Society of Health-System Pharmacists®, 4500 East-West Highway, Suite 900, Bethesda, Maryland 20814.
ReferencesFor a list of references cited in the text of this monograph, search the monograph titled References.