Nafcillin sodium is a white to yellowish-white powder.2801; 2808 Intact vials and pharmacy bulk packages should be stored at controlled room temperature.2801; 2808
Solutions reconstituted with sterile water for injection or sodium chloride 0.9% are stated to be stable for 24 hours at room temperature (25°C) or 7 days under refrigeration (5°C) at concentrations of 10 to 200 mg/mL, or 90 days frozen at -15°C at a concentration of 250 mg/mL.2801 Reconstituted solutions prepared from pharmacy bulk packages should be further diluted for infusion within 4 hours after initial entry into the vial.2808 Unused portions of pharmacy bulk packages should be discarded after 4 hours of initial entry.2808
Solutions further diluted for infusion are stated to be stable for 24 hours at room temperature or 7 days under refrigeration for the following diluents and concentrations: Sterile water for injection or sodium chloride 0.9% at 10 to 200 mg/mL; dextrose 5% or dextrose 5% in sodium chloride 0.45% at 2 to 30 mg/mL; sodium lactate 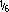 M at 30 mg/mL; and Ringer's injection, lactated at 10 to 30 mg/mL.2801; 2808
M at 30 mg/mL; and Ringer's injection, lactated at 10 to 30 mg/mL.2801; 2808
Commercially-available frozen premixed solutions in single-dose Galaxy containers should be stored at -20°C and thawed at room temperature (25°C) or under refrigeration (5°C).2809 Do not force thaw by immersion in water baths or microwaving.2809 Thawed solutions are stable for 72 hours at room temperature or 21 days under refrigeration.2809 Thawed solutions should not be refrozen.2809 The manufacturer states that precipitation may occur in the frozen solution which will dissolve with little or no agitation upon reaching room temperature.2809 The container should be agitated upon reaching room temperature.2809 Discard the solution if it is cloudy or precipitate is present.2809
pH Effects
The stability of nafcillin sodium is pH dependent, with a maximum stability at pH 6 and a preferred range of pH 5 to 8. Drug decomposition is increased as pH values vary from this range. Additives that may result in a final pH of above 8 or below 5 should not be mixed with nafcillin sodium.27
Freezing Solutions
Reconstituted solutions at a concentration of 250 mg/mL in sterile water for injection or sodium chloride 0.9% are stated to be stable for 90 days frozen at -15°C.2801; 2808 Solutions at concentrations of 10 to 250 mg/mL in dextrose 5%; dextrose 5% in sodium chloride 0.45%; sodium lactate 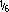 M; and Ringer's injection, lactated are stated to be stable for 90 days frozen at -15°C.2801; 2808
M; and Ringer's injection, lactated are stated to be stable for 90 days frozen at -15°C.2801; 2808
In one study, however, when nafcillin sodium (Wyeth) 1 g/4 mL was frozen at -20°C in glass syringes (Hy-Pod), the drug was stable for nine months.532
In another study, nafcillin sodium (Wyeth) 1 g/50 mL of dextrose 5% in PVC bags frozen at -20°C for 30 days and then thawed by exposure to ambient temperature or microwave radiation showed no evidence of precipitation or color change but had a 2 to 3% loss. Subsequent storage of the admixture at room temperature for 24 hours also yielded physically compatible solutions with no additional loss of activity.555
Syringes
Nafcillin sodium (Apothecon) 10 mg/mL in sodium chloride 0.9% was packaged in 10-mL polypropylene syringes (Becton Dickinson) and stored at 5 and 25°C. The solutions remained clear under refrigeration for 44 days and at room temperature for seven days. A yellow color developed after 14 days at room temperature. About 2% loss of nafcillin sodium occurred after seven days and 18% loss in 14 days at 25°C. Under refrigeration, 1% loss occurred after 44 days. Stability in glass containers was comparable.2325
Ambulatory Pumps
Nafcillin sodium (Marsam) 20 mg/mL in sterile water for injection in PVC portable pump reservoirs (Pharmacia Deltec) exhibited no loss in three days stored at 25°C and in 14 days at 5°C. However, at a concentration of 120 mg/mL, 6% loss was found in three days at 25°C, and 2% loss occurred in 14 days at 5°C.2080
Nafcillin sodium 40 and 50 mg/mL in sodium chloride 0.9% precipitated in as little as one day at the simulated ambulatory use temperature of 35°C. At 20 mg/mL, precipitation appeared in three days. When stored at room temperature, no precipitation appeared at any concentration in three days.2664