AHFS Class:
8:12.16.08 Aminopenicillins
Generic Name
- Ampicillin Sodium-Sulbactam Sodium (CAS Registry Number: 69-52-3)
- Ampicillin Sodium-Sulbactam Sodium (CAS Registry Number: 69388-84-7)
Ampicillin sodium-sulbactam sodium is available as a powder in vials and piggyback bottles containing 1.5 g (ampicillin 1 g plus sulbactam 0.5 g) or 3 g (ampicillin 2 g plus sulbactam 1 g) as the sodium salts.3285 Ampicillin sodium-sulbactam sodium also is available in a pharmacy bulk package containing 15 g (ampicillin 10 g plus sulbactam 5 g as the sodium salts).4007
For intramuscular injection, reconstitute vials with sterile water for injection or lidocaine hydrochloride 0.5 or 2% in the following amounts:3285
| Vial Size | Volume of Diluent | Withdrawable Volume | Concentration |
|---|
| 1.5 g | 3.2 mL | 4 mLa | 375 mg/mLb |
| 3 g | 6.4 mL | 8 mLa | 375 mg/mLb |
aSufficient excess is present to permit withdrawal of the volume noted.bAmpicillin 250 mg plus sulbactam 125 mg per mL.
For intravenous use, ampicillin sodium-sulbactam sodium may be reconstituted with sterile water for injection; sodium chloride 0.9%; dextrose 5%; Ringer's injection, lactated; sodium lactate 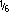 M, or dextrose 5% in sodium chloride 0.45%.3285 Reconstitute piggyback bottles directly with a compatible diluent to the desired concentration between 3 and 45 mg/mL (ampicillin 2 to 30 mg plus sulbactam 1 to 15 mg per mL).3285 Standard vials of 1.5 and 3 g may be reconstituted with 3.2 and 6.4 mL of sterile water for injection, respectively, to yield 375-mg/mL solutions (ampicillin 250 mg plus sulbactam 125 mg per mL).3285 The reconstituted solution should be diluted immediately in a compatible infusion solution to yield the desired concentration between 3 and 45 mg/mL.3285 Allow reconstituted solution to stand so that any foaming may dissipate before inspecting them visually to ensure complete dissolution.3285
M, or dextrose 5% in sodium chloride 0.45%.3285 Reconstitute piggyback bottles directly with a compatible diluent to the desired concentration between 3 and 45 mg/mL (ampicillin 2 to 30 mg plus sulbactam 1 to 15 mg per mL).3285 Standard vials of 1.5 and 3 g may be reconstituted with 3.2 and 6.4 mL of sterile water for injection, respectively, to yield 375-mg/mL solutions (ampicillin 250 mg plus sulbactam 125 mg per mL).3285 The reconstituted solution should be diluted immediately in a compatible infusion solution to yield the desired concentration between 3 and 45 mg/mL.3285 Allow reconstituted solution to stand so that any foaming may dissipate before inspecting them visually to ensure complete dissolution.3285
The 15-g pharmacy bulk package should be reconstituted with 92 mL of sterile water for injection or sodium chloride 0.9% to yield a solution containing ampicillin 100 mg and sulbactam 50 mg per mL.4007 Add the diluent in 2 separate aliquots (e.g., 50 mL followed by 42 mL) and shake after addition of each aliquot.4007 Allow reconstituted solution to stand so that any foaming may dissipate to permit visual inspection for complete dissolution.4007 The reconstituted solution must be further diluted for intravenous infusion.4007
pH
Reconstituted solutions and diluted solutions: 8 to 10.3285; 4007
Sodium Content
Each 1.5 g (ampicillin 1 g plus sulbactam 0.5 g as the sodium salts) contains approximately 115 mg (5 mEq) of sodium.3285
Each 3 g (ampicillin 2 g plus sulbactam 1 g as the sodium salts) contains approximately 230 mg (10 mEq) of sodium.3285
Trade Name(s)
Unasyn
Ampicillin sodium-sulbactam sodium may be administered by deep intramuscular injection or intravenous injection or infusion.3285 For direct intravenous injection, the drug should be given slowly over at least 10 to 15 minutes.3285 For intravenous infusion, the drug may be diluted in 50 to 100 mL of compatible diluent and infused over 15 to 30 minutes.3285
Intact vials should be stored at or below 30°C.3285; 4007 Ampicillin sodium-sulbactam sodium is available as a white to off-white powder that forms a pale yellow to yellow solution upon reconstitution.3285; 4007 Dilute solutions are colorless to pale yellow.3285; 4007 The manufacturer recommends that intramuscular solutions be used within 1 hour after preparation.3285
Reconstituted solutions prepared from pharmacy bulk packages should be further diluted for intravenous infusion within 2 hours if stored at room temperature (20°C) or within 4 hours if stored under refrigeration.4007 If the reconstituted bulk solution has been stored for less than 1 hour at room temperature, resulting diluted solutions should be used within the time periods indicated in Table 1.4007 If the reconstituted bulk solution has been stored for 1 to 2 hours at room temperature, resulting diluted solutions should be used within the time periods indicated in Table 2.4007
Table 1: Use Periods for Diluted Ampicillin Sodium-Sulbactam Sodium Solutions Prepared from Reconstituted Bulk Solutions That Have Been Stored 1 Hour at Room Temperature4007
| Diluent | Maximum Concentration
(ampicillin/sulbactam)
(mg/mL) | Storage Temperature
21°C | Storage Temperature
4°C |
|---|
| Sterile water for injection | 45 (30/15) | 8 hours | 48 hours |
| Sterile water for injection | 30 (20/10) | | 72 hours |
| Sodium chloride 0.9% | 45 (30/15) | 8 hours | 48 hours |
| Sodium chloride 0.9% | 30 (20/10) | | 72 hours |
| Dextrose 5% | 30 (20/10) | 2 hours | 4 hours |
| Dextrose 5% | 3 (2/1) | 2 hours | |
| Ringer’s injection, lactated | 45 (30/15) | 8 hours | 24 hours |
Sodium lactate 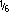 M M | 45 (30/15) | 8 hours | 12 hours |
| Dextrose 5% in sodium chloride 0.45% | 3 (2/1) | 4 hours | |
| Dextrose 5% in sodium chloride 0.45% | 15 (10/5) | | 4 hours |
Table 2: Use Periods for Diluted Ampicillin Sodium-Sulbactam Sodium Solutions Prepared from Reconstituted Bulk Solutions That Have Been Stored 1-2 Hours at Room Temperature4007
| Diluent | Maximum Concentration
(ampicillin/sulbactam)
(mg/mL) | Storage Temperature
21°C | Storage Temperature
4°C |
|---|
| Sterile water for injection | 45 (30/15) | 4 hours | 24 hours |
| Sodium chloride 0.9% | 45 (30/15) | 4 hours | 24 hours |
Central Venous Catheter
Ampicillin sodium-sulbactam sodium (Pfizer-Roerig) 5 plus 2.5 mg/mL in sodium chloride 0.9% was found to be compatible with the ARROWg+ard Blue Plus (Arrow International) chlorhexidine-bearing triple-lumen central catheter. Essentially complete delivery of the drug was found with little or no drug loss occurring. Furthermore, chlorhexidine delivered from the catheter remained at trace amounts with no substantial increase due to the delivery of the drug through the catheter.2335
References
For a list of references cited in the text of this monograph, search the monograph titled References.
ASHP® Injectable Drug InformationTM. Selected Revisions September 12, 2025. © Copyright, 2025. American Society of Health-System Pharmacists®, 4500 East-West Highway, Suite 900, Bethesda, Maryland 20814.
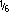 M, or dextrose 5% in sodium chloride 0.45%.3285 Reconstitute piggyback bottles directly with a compatible diluent to the desired concentration between 3 and 45 mg/mL (ampicillin 2 to 30 mg plus sulbactam 1 to 15 mg per mL).3285 Standard vials of 1.5 and 3 g may be reconstituted with 3.2 and 6.4 mL of sterile water for injection, respectively, to yield 375-mg/mL solutions (ampicillin 250 mg plus sulbactam 125 mg per mL).3285 The reconstituted solution should be diluted immediately in a compatible infusion solution to yield the desired concentration between 3 and 45 mg/mL.3285 Allow reconstituted solution to stand so that any foaming may dissipate before inspecting them visually to ensure complete dissolution.3285
M, or dextrose 5% in sodium chloride 0.45%.3285 Reconstitute piggyback bottles directly with a compatible diluent to the desired concentration between 3 and 45 mg/mL (ampicillin 2 to 30 mg plus sulbactam 1 to 15 mg per mL).3285 Standard vials of 1.5 and 3 g may be reconstituted with 3.2 and 6.4 mL of sterile water for injection, respectively, to yield 375-mg/mL solutions (ampicillin 250 mg plus sulbactam 125 mg per mL).3285 The reconstituted solution should be diluted immediately in a compatible infusion solution to yield the desired concentration between 3 and 45 mg/mL.3285 Allow reconstituted solution to stand so that any foaming may dissipate before inspecting them visually to ensure complete dissolution.3285