AHFS Class:
91:04.12 Chemotherapy antidotes/protectants
Dexrazoxane hydrochloride (Zinecard) is available as a lyophilized powder in single-dose vials containing the equivalent of 250 or 500 mg of dexrazoxane.3392 Reconstitution of the contents of the 250- or 500-mg Zinecard vial with 25 or 50 mL, respectively, of sterile water for injection results in a solution with a dexrazoxane concentration of 10 mg/mL.3392 The pH has been adjusted during manufacturing with hydrochloric acid.3392 The reconstituted solution should be diluted in an infusion bag with Ringer’s injection, lactated to a final dexrazoxane concentration of 1.3 to 3 mg/mL.3392
Dexrazoxane hydrochloride (generic; Mylan) also is available as a lyophilized powder in single-use vials containing the equivalent of 250 or 500 mg of dexrazoxane.3393 Each vial is packaged with a 25- or 50-mL vial, respectively, of 0.167 M (i.e., 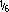 M) sodium lactate for use as a diluent for reconstituting the drug.3393 Reconstitution of the contents of the 250- or 500-mg vials with 25 or 50 mL, respectively, of the provided diluent results in a solution with a dexrazoxane concentration of 10 mg/mL.3393 The pH has been adjusted during manufacturing with hydrochloric acid.3393 The reconstituted solution may be transferred to an empty infusion bag or may be diluted in an infusion bag with sodium chloride 0.9% or dextrose 5% to a final dexrazoxane concentration of 1.3 to 5 mg/mL.3393
M) sodium lactate for use as a diluent for reconstituting the drug.3393 Reconstitution of the contents of the 250- or 500-mg vials with 25 or 50 mL, respectively, of the provided diluent results in a solution with a dexrazoxane concentration of 10 mg/mL.3393 The pH has been adjusted during manufacturing with hydrochloric acid.3393 The reconstituted solution may be transferred to an empty infusion bag or may be diluted in an infusion bag with sodium chloride 0.9% or dextrose 5% to a final dexrazoxane concentration of 1.3 to 5 mg/mL.3393
Dexrazoxane hydrochloride (Totect) is available as a lyophilized powder in single-dose vials containing the equivalent of 500 mg of dexrazoxane.3394 Reconstitution of the contents of the 500-mg Totect vial with 50 mL of 0.167 M sodium lactate results in a solution with a dexrazoxane concentration of 10 mg/mL.3394 The resulting solution also contains hydrochloric acid, sodium hydroxide, and lactic acid in water for injection.3394 The appropriate volume of the reconstituted solution should be diluted in 1 L of sodium chloride 0.9%.3394
pH
The pH of reconstituted Zinecard solutions ranges from 1 to 3 while the pH of Zinecard diluted for infusion ranges from 3.5 to 5.5.3392
The pH of reconstituted solutions of dexrazoxane (generic; Mylan) ranges from 3.5 to 5.5.3393
Trade Name(s)
Totect, Zinecard
Diluted solutions of Zinecard for infusion should be administered by rapid intravenous drip infusion over 15 minutes.3392
The reconstituted solution of dexrazoxane (generic; Mylan) is administered by slow intravenous push; alternatively, the reconstituted solution may be diluted and administered by rapid intravenous drip infusion.3393
Diluted solutions of Totect for infusion should be administered by intravenous infusion over 1 to 2 hours at room temperature and under normal light conditions into a large caliber vein in an extremity or area other than the one affected by the anthracycline extravasation.3394 Any cooling methods (e.g., ice packs) used for the extravasation being treated should be removed from the area at least 15 minutes prior to Totect administration.3394
As with other toxic drugs, caution should be exercised in the handling and preparation of dexrazoxane and applicable special handling and disposal procedures should be followed.3392; 3393; 3394 Manufacturers recommend the use of gloves.3392; 3393; 3394 If skin contact with the drug occurs, the affected area(s) should be washed immediately and thoroughly with soap and water.3392; 3393; 3394
Intact vials of dexrazoxane should be stored at controlled room temperature.3392; 3393; 3394 One manufacturer recommends that Totect vials remain in the original carton to protect from light.3394
Reconstituted solutions of Zinecard are stable for 30 minutes at room temperature or for up to 3 hours at 2 to 8°C.3392 Diluted solutions of Zinecard for infusion are stable for 1 hour at room temperature or for up to 4 hours at 2 to 8°C.3392
The manufacturer states that reconstituted solutions of dexrazoxane (generic; Mylan) are stable for 6 hours from the time of reconstitution when stored in an empty infusion bag at controlled room temperature of 20 to 25°C or at 2 to 8°C; this preparation of dexrazoxane diluted for infusion in sodium chloride 0.9% or dextrose 5% is stable for 6 hours at 20 to 25°C or 2 to 8°C.3393
Reconstituted solutions of Totect should be diluted within 2 hours after reconstitution.3394 Diluted solutions of Totect for infusion should be used immediately after preparation, but are stable for 4 hours from the time of preparation when stored below 25°C.3394
Solutions should be visually inspected for particulate matter and discoloration prior to administration; solutions containing a precipitate should be discarded.3392; 3393; 3394 Any unused portions should be discarded.3392; 3393; 3394
In one study, reconstituted solutions of dexrazoxane (National Cancer Institute) 10 mg/mL were found to be stable for about 24 hours at 20 to 22°C exposed to or protected from fluorescent light; about 7% loss occurred when reconstituted with dextrose 5% and about 9% loss occurred when reconstituted with sodium chloride 0.9%.2395 Refrigeration resulted in precipitation after 1 day.2395
In another study, reconstituted solutions of Zinecard 10 mg/mL prepared with sterile water for injection and dexrazoxane (generic; Mylan) prepared with 0.167 M sodium lactate were stable for 8 hours at room temperature; the reconstituted solutions exhibited a mean drug loss of 11 and 13%, respectively, at 24 hours.3395
pH Effects
Dexrazoxane is most stable at acidic pH and is highly unstable in alkaline solutions.2395 Dexrazoxane degrades rapidly at pH values above 7;3392; 3393 more than 10% loss occurred in less than 2 hours at such pH values.2395
Light Effects
No adverse effect on drug stability was found in 1- and 10-mg/mL solutions when exposed to fluorescent light.2395
References
For a list of references cited in the text of this monograph, search the monograph titled References.
ASHP® Injectable Drug InformationTM. Selected Revisions December 5, 2024. © Copyright, 2025. American Society of Health-System Pharmacists®, 4500 East-West Highway, Suite 900, Bethesda, Maryland 20814.
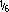 M) sodium lactate for use as a diluent for reconstituting the drug.3393 Reconstitution of the contents of the 250- or 500-mg vials with 25 or 50 mL, respectively, of the provided diluent results in a solution with a dexrazoxane concentration of 10 mg/mL.3393 The pH has been adjusted during manufacturing with hydrochloric acid.3393 The reconstituted solution may be transferred to an empty infusion bag or may be diluted in an infusion bag with sodium chloride 0.9% or dextrose 5% to a final dexrazoxane concentration of 1.3 to 5 mg/mL.3393
M) sodium lactate for use as a diluent for reconstituting the drug.3393 Reconstitution of the contents of the 250- or 500-mg vials with 25 or 50 mL, respectively, of the provided diluent results in a solution with a dexrazoxane concentration of 10 mg/mL.3393 The pH has been adjusted during manufacturing with hydrochloric acid.3393 The reconstituted solution may be transferred to an empty infusion bag or may be diluted in an infusion bag with sodium chloride 0.9% or dextrose 5% to a final dexrazoxane concentration of 1.3 to 5 mg/mL.3393