Bone and Soft-Tissue Disorders
= malignant tumor of connective tissue producing osteoid matrix + variable amounts of cartilage matrix + fibrous tissue
Most common malignant primary bone tumor in adolescents + children; 2nd most common primary malignant bone tumor after multiple myeloma
Prevalence: 4–5÷1,000,000 annually; 15% of all primary bone tumors confirmed at biopsy; <1% of all cancers in USA
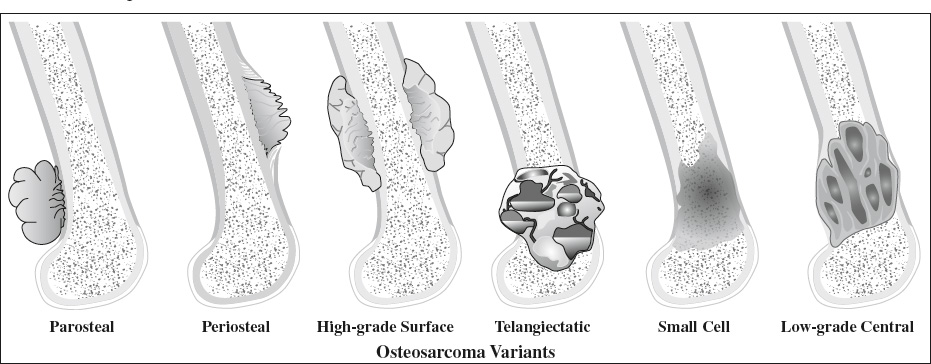
Types & Frequency: underlined are types recognized by WHO
- Conventional osteosarcoma:
- high-grade intramedullary 75%
- telangiectatic 4.5–11%
- low-grade intraosseous 4–5%
- small cell 1–4%
- osteosarcomatosis 3–4%
- gnathic 6–9%
- Surface / juxtacortical osteosarcoma: 4–10%
associated with periosteum + variable medullary canal involvement- parosteal 65%
- periosteal 25%
- high-grade surface 10%
- intracortical rare
- Extraskeletal 4%
- Secondary osteosarcoma 5–7%
Work-up: local staging by MR before biopsy; distant staging with bone scan + chest CT
Prognosis: dependent on age, sex, tumor size, site, classification; best predictor is degree of tissue necrosis in postresection specimen following chemotherapy (91% survival with tumor necrosis >90%, 14% survival with <90% tumor necrosis)
= extremely uncommon high-grade malignant tumor that occurs in older age group than osteosarcoma of bone
Frequency: 1.2% of soft-tissue sarcomas
Histo: variable amounts of neoplastic osteoid + bone + cartilage; frequently associated with fibrosarcoma, malignant fibrous histiocytoma, malignant peripheral nerve sheath tumor
Mean age: 50 years; 94% >30 years of age; M >F
Location: lower extremity (thigh in 42–47%); upper extremity (12–23%); retroperitoneum (8–17%); buttock, back, orbit, submental, axilla, abdomen, neck, cheek, parotid gland, scalp, soft tissues adjacent to mandible, kidney, breast
Site: in soft tissue without attachment to bone / periosteum
- slowly growing firm soft-tissue mass; painful + tender (in 25–50%)
- history of trauma (12–31%): in preexisting myositis ossificans / site of intramuscular injection
- history of irradiation (5–10%)
- elevated levels of alkaline phosphatase (prognostic)
Size: average diameter of 9 cm
- well circumscribed often deep-seated and fixed tumor
- focal / massive area of characteristically amorphous mineralization (>50%) most prominent at center of lesion
- faint moderate inhomogeneous enhancement
- increased radionuclide uptake on bone scan
Prognosis:
- multiple local recurrences (in 80–90%) after interval of 2 months to 10 years
- metastases after interval of 1 month to 4 years: lungs (81–100%), lymph nodes (25%), bone, subcutis, liver
- death within 2–3 years (>50%) with tumor size as major predictor
DDx: myositis ossificans (calcifications most prominent at periphery of lesion)
High-grade Intramedullary Osteosarcoma
= CENTRAL / CONVENTIONAL OSTEOSARCOMA
Histo: arising from undifferentiated mesenchymal tissue; forming fibrous / cartilaginous / osseous matrix (mostly mixed) that produces osteoid / immature bone
- osteoblastic (50–80%)
- chondroblastic (5–25%)
- fibroblastic-fibrohistiocytic (7–25%)
Age: bimodal distribution 10–25 years and >60 years; 21% <10 years; 68% <15 years; 70% between 10 and 30 years; M÷F = 3÷2 to 2÷1; >35 years: related to preexisting condition
- painful swelling (1–2 months' duration); fever (frequent)
- slight elevation of alkaline phosphatase
- diabetes mellitus (paraneoplastic syndrome) in 25%
Location: long bones (70–80%), femur (40–45%), tibia (16–20%); 50–55% about knee; proximal humerus (10–15%); facial bones (8%); cylindrical bone <30 years; flat bone (ilium) >50 years; spine (0.6–3.2%)
Site: origin in metaphysis (90–95%) / diaphysis (2–11%) /epiphysis (<1%); growth through open physis with extension into epiphysis (75–88%)
Doubling time: 20–30 days
- usually large bone lesion of >5–6 cm when first detected
- cloudlike density (90%) / almost normal density / osteolytic (fibroblastic type)
- aggressive periosteal reaction: sunburst / hair-on-end / onion-peel = laminated / Codman triangle
- moth-eaten bone destruction + cortical disruption
- soft-tissue mass with tumor new bone (osseous / cartilaginous type)
- transphyseal spread before plate closure (75–88%); physis does NOT act as a barrier to tumor spread
- spontaneous pneumothorax ← subpleural metastases
NUC (bone scintigraphy):
- intensely increased activity on blood flow, blood pool, delayed images (hypervascularity, new-bone formation)
- soft-tissue extension demonstrated, especially with SPECT
- bone scan establishes local extent (extent of involvement easily overestimated due to intensity of uptake), skip lesions, metastases to bone + soft tissues
CT:
- very high attenuation (mineralized matrix) in 80%
- soft-tissue attenuation (nonmineralized portion) replacing fatty bone marrow
- low attenuation (higher water content of chondroblastic component / hemorrhage / necrosis)
MR (preferred modality):
- tumor of intermediate SI on T1WI + high SI on T2WI
- clearly defines marrow extent (best on T1WI), vascular involvement, soft-tissue component (best on T2WI)
Evaluate for:
- extent of marrow + soft-tissue involvement
- invasion of epiphysis
- joint (19–24%) + neurovascular involvement
- viable tumor + mineralized matrix for biopsy
Metastases (in 2% at presentation):
- hematogenous lung metastases (15%): calcifying; spontaneous pneumothorax ← subpleural cavitating nodules rupturing into pleural space
- lymph nodes, liver, brain (may be calcified)
- skeletal metastases uncommon (unlike Ewing sarcoma); skip lesions = discontinuous tumor foci in marrow cavity in 1–25%
Cx:
- pathologic fracture (15–20%)
- radiation-induced osteosarcoma (30 years delay)
Rx: chemotherapy followed by wide surgical resection
Prognosis: 60–80% 5-year survival
- Amputation: 20% 5-year survival; 15% develop skeletal metastases; 75% dead within <2 years
- Multidrug chemotherapy: 55% 4-year survival more proximal lesions carry higher mortality (0% 2-year survival for axial primary)
Predictors of poor outcome:
metastasis at presentation, soft-tissue mass >20 cm, pathologic fracture, skip lesions in marrow
Predictors of poor response to chemotherapy:
no change / increase in size of soft-tissue mass, increase in bone destruction
DDx: Osteoid osteoma, sclerosing osteomyelitis, Charcot joint
High-grade Surface Osteosarcoma
Frequency: 0.4% of all osteosarcomas; least common of juxtacortical osteosarcomas
Histo: entirely high-grade mitotic activity
Origin: surface of bone
Age: 2nd + 3rd decades
Location: femur >humerus, fibula
Site: diaphysis + metaphysis of long bone
Size: usually 4.5–22 cm large tumor
- dense ossification + periosteal reaction similar to periosteal osteosarcoma with bulk of lesion external to bone
- cortical erosion + thickening (frequent)
- often involves entire circumference of bone
- invasion of medullary canal (in 8–48%)
Prognosis: 5-year survival rate of 46% (slightly better than conventional osteosarcoma)
DDx:
- Parosteal osteosarcoma (ill-defined fluffy bone formation)
- Periosteal osteosarcoma (diaphysis, cortical destruction, periosteal reaction)
- Conventional osteosarcoma
Rarest form of osteosarcoma
Histo: sclerosing variant of osteosarcoma which may contain small foci of chondro- or fibrosarcoma
Location: femur, tibia
- tumor <4 cm in diameter
- intracortical geographic bone lysis
- tumor margin may be well defined with thickening of surrounding cortex
- metastases in 29%
Low-grade Intraosseous Osteosarcoma
= LOW-GRADE CENTRAL OSTEOSARCOMA = WELL-DIFFERENTIATED / SCLEROSING OSTEOSARCOMA
Frequency:<1% of all osteosarcomas
Path: penetration among bony trabeculae; fibrous stroma sometimes lacking nuclear atypia + pleomorphism; permeative extension of tumor cells between mature bone trabeculae
Histo: microtrabecular osseous matrix in bland stroma with highly variable amount of tumor osteoid production (similar to fibro-osseous lesions) = equivalent to low-grade parosteal osteosarcoma
Age: most frequently 3rd + 4th decade; M÷F = 1÷1
- protracted clinical course with nonspecific symptoms
Location: about the knee; femur involved in 50%
Site: medullary canal of metaphysis; often with extension into epiphysis
- variable radiographic features:
- expansile lytic bone destruction with coarsely thick / thin trabeculations (61%)
- diffuse dense sclerosis (<30%)
- remodeling of bone
- may have well-defined margins + sclerotic rim
- subtle signs of aggressiveness: bone lysis, focally indistinct margin, cortical destruction, soft-tissue mass, variable periosteal reaction (22–50%)
N.B.: the relatively benign appearance has resulted in misdiagnosis as a benign entity!
Cx: transformation into high-grade osteosarcoma
Rx: surgical resection alone
Prognosis: 80–90% 5-year survival rate, similar to parosteal osteosarcoma; local recurrence in 10% (due to inadequate resection)
DDx: benign fibro-osseous lesions (fibrous dysplasia, nonossifying fibroma, desmoplastic fibroma, chondromyxoid fibroma); chondrosarcoma
= GNATHIC OSTEOSARCOMA
Average age: 34 years (10–15 years older than in conventional osteosarcoma)
Histo: chondroblastic predominance (~50%), osteoblastic predominance (~25%); better differentiated (grade 2 or 3) than conventional osteosarcoma (grade 3 or 4)
- simulating periodontal disease: rapidly enlarging mass, lump, swelling
- paresthesia (if inferior alveolar nerve involved)
- painful / loose teeth, bleeding gum
Location: body of mandible (lytic), alveolar ridge of maxilla (sclerotic), maxillary antrum
- osteolytic / osteoblastic / mixed pattern
- osteoid matrix (60–80%)
- aggressive periosteal reaction for mandibular lesion
- soft-tissue mass (100%)
- opacification of maxillary sinus (frequent in maxillary lesions)
Prognosis: 40% 5-year survival rate (lower probability of metastases, lower grade)
DDx: metastatic disease (lung, breast, kidney), multiple myeloma, direct invasion by contiguous tumor from oral cavity, Ewing sarcoma, primary lymphoma of bone, chondrosarcoma, fibrosarcoma, acute osteomyelitis, ameloblastoma, Langerhans cell histiocytosis, giant cell reparative granuloma, “brown tumor” of HPT
= MULTIFOCAL OSTEOSARCOMA = MULTIPLE SCLEROTIC OSTEOSARCOMA
Frequency: 2.7–4.2% of osteosarcomas
Etiology:
- multicentric type of osteosarcoma
- multiple metastatic bone lesions
Classification (Amstutz):
- Type I multiple synchronous bone lesions occurring within 5 months of diagnosis + patient ≤18 years of age
- Type II multiple synchronous bone lesions occurring within 5 months of presentation + patient >18 years of age
- Type IIIa early metachronous metastatic osteosarcoma occurring 5 to 24 months after diagnosis
- Type IIIb late metachronous metastatic osteosarcoma occurring >24 months after diagnosis
Mean age: Amstutz type I = 11 (range, 4–18) years Amstutz type II = 30 (range, 19–63) years
Site: metaphysis of long bones; may extend into epiphyseal plate / begin in epiphysis
- multicentric simultaneously appearing lesions with a radiologically dominant tumor (97%)
- smaller lesions are densely opaque (osteoblastic)
- lesions bilateral + symmetrical
- early: bone islands
- late: entire metaphysis fills with sclerotic lesions breaking through cortex
- lesions are of same size
- lung metastases (62%)
Prognosis: uniformly poor with mean survival of 12 (range, 6–37) months
DDx: heavy metal poisoning, sclerosing osteitis, progressive diaphyseal dysplasia, melorheostosis, osteopoikilosis, bone infarction, osteopetrosis
Frequency: 4–5% of all osteosarcomas; 65% of all juxtacortical osteosarcomas
Origin: outer fibrous layer of periosteum; slowly growing lesion with fulminating course if tumor reaches medullary canal
Histo: low-grade lesion with higher-grade regions (22–64%), invasion of medullary canal (8–59%); fibroblastic stroma + extensive osteoid with small foci of cartilage
Age: peak age 38 years (range of 12–58 years); 50% >age 30 (for central osteosarcoma 75% <age 30); M÷F = 2÷3
Location: posterior aspect of distal femur (50–65%), either end of tibia, proximal humerus, fibula, rare in other long bones
Site: metaphysis of long bones (80–90%)
- palpable mass
- large lobulated “cauliflower-like” homogeneously ossified exophytic mass extending away from cortex
- “string” sign = initially fine radiolucent cleavage plane separating tumor mass from cortex (30–40%)
- tumor stalk (= attachment to cortex) grows with tumor obliterating the radiolucent cleavage plane
- cortical thickening without aggressive periosteal reaction
- tumor periphery less dense than center (DDx: myositis ossificans with periphery more dense than center + without attachment to cortex)
- large soft-tissue component with osseous + cartilaginous elements
MR:
- predominantly low SI on T1WI + T2WI = ossified tumor
- unmineralized soft-tissue mass >1 cm3 / predominantly high T2-SI suggests high-grade tumor component
Prognosis: 86–91% 5-year survival rate (best prognosis of all osteosarcomas) compared to 53–61% for conventional osteosarcoma; dedifferentiation from low to high grade (in 16–43%)
DDx:
- Osteochondroma (corticomedullary continuity)
- Myositis ossificans, periosteal chondroma, juxtacortical hematoma, fibrous malignancy, periosteal chondrosarcoma, other subtypes of juxtacortical osteosarcomas
Frequency: 1.5% of all osteosarcomas; 2nd most common of juxtacortical osteosarcomas
Origin: deep inner germinative layer of periosteum
Histo: intermediate-grade lesion; highly chondroblastic lesion with smaller areas of osteoid formation
Average age: 20 (range, 3–70) years); M÷F = 1.7÷1
Location: tibia (40%), femur (38%), ulna + humerus (5–10%)
Site: anteromedial diaphysis of proximal tibia + middle / distal femur; limited to periphery of cortex with normal endosteal margin + medullary canal (resembles parosteal sarcoma)
- broad-based soft-tissue mass attached to cortex over entire extent of tumor (100%):
- tumor 7–12 cm in length, 2–4 cm in width
- involving 50–55% of osseous circumference
- cortical thickening (82%): solid nonaggressive (51%)
- cortical erosion
- extrinsic scalloping of cortex (92%):
- affecting only thickened cortex (68%)
- involving native cortex (32%)
- periosteal reaction (95%):
- short spicules of new bone perpendicular to shaft extending into soft-tissue mass (51%)
- aggressive periosteal reaction of laminated appearance / Codman triangle (11%)
- both patterns (38%)
- cortical destruction / medullary cavity invasion (rare):
- marrow signal abnormality on MR usually due to reactive changes – unless continuous with surface component (2%)
- additional areas of matrix calcification by CT (91%)
- chondroblastic areas (80%) with inherent high water content of hyaline cartilage:
- hypodense on CT compared to muscle (91%)
- very high signal intensity on T2WI (83%)
- Biopsy may lead to erroneous diagnosis of chondrosarcoma!
NUC (bone scintigraphy):
- eccentric uptake (100%)
Prognosis: 83% 5-year survival rate (better than for conventional osteosarcoma but worse than for parosteal osteosarcoma)
DDx:
- Juxtacortical chondrosarcoma (4th–5th decade, extensive osteoid + chondroid mineralization, no perpendicular periosteal reaction)
- Ewing sarcoma (rarely periosteal, no perpendicular periosteal reaction, soft-tissue component not mineralized + not low in attenuation + not of very high intensity)
- Parosteal osteosarcoma (densely ossified juxtacortical mass, 3rd + 4th decade, posterior distal metaphysis of femur, attached to bone by narrow stalk, no perpendicular periosteal reaction)
- High-grade surface osteosarcoma (surrounds >50% of bone circumference, frequent invasion of medullary cavity, no high water content of soft-tissue mass)
- Periosteal chondroid tumor (well-defined border, typically metaphyseal location, curvilinear peripheral calcifications)
= lesion arising from a preexisting abnormality
◊Most osteosarcomas in patients >age 60 are secondary!
Cause: malignant transformation of benign process
- Paget disease (67–90%)
◊ 0.2–7.5% of patients with Paget disease develop osteosarcoma dependent on extent of disease - Sequelae of irradiation (6–22%) 2–40 years ago (malignant fibrous histiocytoma most common; fibrosarcoma 3rd most common)
◊ 0.02–4% of patients with radiation therapy develop osteosarcoma related to exposure dose (usually >1,000 cGy) - Osteonecrosis, fibrous dysplasia, metallic implants, osteogenesis imperfecta, chronic osteomyelitis, retinoblastoma (familial bilateral type)
Path: high-grade anaplastic tissue with little / no mineralization
Age: middle-aged / late adulthood
Location: thoracic + lumbar spine >sacrum >cervical spine
Site: posterior elements (79%), 2 vertebral levels (17%)
- aggressive bone destruction in area of preexisting condition associated with large soft-tissue mass
Prognosis:<5% 5-year survival rate
Frequency: 1% of all osteosarcomas
Age: 2nd + 3rd decade; M÷F = 1÷1
Histo: small round blue cells (similar to Ewing sarcoma / primitive neuroectodermal tumor) lacking cellular uniformity and consistently producing fine reticular osteoid
Location: distal femur
Site:metaphysis with frequent extension into epiphysis; diaphysis (in 15%)
- permeative lytic medullary lesion in all cases
- cortical breakthrough
- aggressive periosteal reaction (>50%)
- associated soft-tissue mass
Prognosis: 53–61% 5-year survival rate
DDx:
- Ewing sarcoma (rare calcifications, cortical thickening + saucerization)
- Lymphoma (spread outside bone without osseous destruction, calcification uncommon)
- Conventional osteosarcoma
= MALIGNANT BONE ANEURYSM
Frequency: 1.2–12% of all osteosarcomas
Mean age: 20 (range, 3–67) years; M÷F = 3÷2
Path: malignant destructive osteoid-forming sarcoma of bone with >90% of tumor volume consisting of large hemorrhagic + necrotic cavities mimicking an ABC
Histo: blood-filled cavernous vessels lined with osteoclastic giant cells; nuclear pleomorphism + high mitotic rate
Location:
- about knee (62%): distal femur (48%), proximal tibia (14%)
- proximal humerus (16%), proximal femur, fibula, midfemur, midhumerus, mandible
Site: medullary cavity of metaphysis (90%); extension into epiphysis (87%)
- radiolucent appearance of tumor ← scant bone matrix subtly visible radiographically in 58%
- minimal peripheral sclerosis
- geographic bone destruction with a wide zone of transition
- marked aneurysmal expansion of bone (19%)
- endosteal scalloping
- extensive invasion of surrounding soft tissues
MR:
- heterogeneous signal intensity with fluid levels (74%):
- high T1 + variable T2 signal = hemorrhage (96%)
- enhancing thickened nodular tumor periphery + septa
CT:
- soft-tissue mass with attenuation lower than muscle
- fluid levels (49%)
- nodular calcific foci of osteoid matrix mineralization (85%) at periphery / within septa = SPECIFIC ← viable neoplastic tissue
- thick peripheral + nodular septal enhancement ← viable high-grade sarcomatous tissue
NUC:
- “doughnut” sign = peripherally increased uptake with central photopenia on bone scan = TYPICAL
Cx:
- pathologic fracture (43–61%)
Prognosis: 67% 5-year survival rate
DDx:
- Aneurysmal bone cyst (expansile remodeling with well-defined encapsulated margin, thin septa without nodularity, enhancing thin peripheral rim, no soft-tissue involvement)
- Giant cell tumor (epiphyseal location, solid mass, isointense to muscle on T1WI)
- Lytic metastasis(no fluid levels)
- Chondroblastic conventional osteosarcoma
- Ewing sarcoma, chondrosarcoma, lymphoma
- Extraskeletal Osteosarcoma
- High-grade Intramedullary Osteosarcoma
- High-grade Surface Osteosarcoma
- Intracortical Osteosarcoma
- Low-grade Intraosseous Osteosarcoma
- Osteosarcoma of Jaw
- Osteosarcomatosis
- Parosteal Osteosarcoma
- Periosteal Osteosarcoma
- Secondary Osteosarcoma
- Small-cell Osteosarcoma
- Telangiectatic Osteosarcoma