Methods of Administering Local ED Drugs
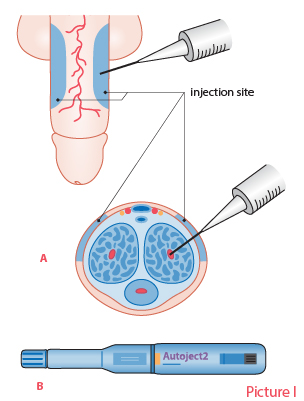
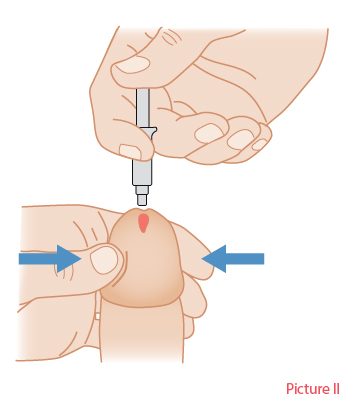
Methods of administering local ED drugs.Picture I. Injectable drugs. Injection technique (A) and auto-injector (Autoject 2) for administering an injectable drug (B). Picture II. Topically administered drug. Introduce some of the cream into the urethra.
Picture I: Piha J. [Steps of pharmacotherapy]. In: Brusila P, Kero K, Piha J et al (eds.). [Sexual Medicine]. Helsinki: Duodecim Publishing Company 2020. Picture II: Duodecim Publishing Company.
Primary/Secondary Keywords
- erectile dysfunction
- ED
- penile erection
- local drug administerion
- injection treatment
- alprostadil