Tests for fetal maturity are generally performed after the 35th week of pregnancy, when preterm delivery is being considered because of fetal or maternal problems. The lungs are the last of the fetal organs to mature; therefore, the most common complication of early delivery is newborn respiratory distress syndrome (RDS). Tests of amniotic fluid for fetal maturity focus on determining fetal lung maturity and include the lecithin:sphingomyelin (L:S) ratio, as well as measures of other lung surface lipids such as phosphatidylglycerol and phosphatidylinositol. If the lungs are found to be mature by these tests, the other body organs also are assumed to be mature.17,18 Tests of amniotic fluid, which can be used to indicate maturity of other fetal organ systems, include creatinine and bilirubin determinations, as well as examination of fetal cells for type and lipid content.
During the last trimester of pregnancy, fetal lung enzyme systems initiate the production of surfactant by type II pneumocytes, which line the alveoli. Surfactant, a phospholipid mixture, lowers the surface tension in the alveoli and prevents them from collapsing during exhalation. The phospholipid components of surfactant are (1) lecithin (phosphatidylcholine), (2) sphingomyelin, (3) phosphatidyl glycerol (PG), (4) phosphatidylethanolamine (PE), (5) phosphatidylinositol (PI), and (6) phosphatidylserine (PS). Surfactant appears in amniotic fluid as a result of fetal respiratory movements that cause it to diffuse from fetal airways.19
Lecithin constitutes about 75 percent of surfactant in mature lungs and is responsible for most of the surface activity of surfactant. The saturated form of lecithin, 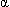 -palmitic
-palmitic 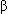 -myristic lecithin, is seen early in the third trimester; the desaturated form, dipalmitic lecithin (L), begins to appear at approximately 35 weeks' gestation and continues to increase throughout the remainder of the pregnancy. Sphingomyelin, a surfactant component without major surface activity properties, remains fairly constant during pregnancy. The L:S ratio measures the relationship between lecithin and sphingomyelin; if the increasing amount of lecithin over the relatively constant amount of sphingomyelin produces a ratio of 2:1 or more, fetal lung maturity is generally indicated, as long as the pregnancy is uncomplicated and the amniotic fluid sample is not contaminated with blood or meconium.20
-myristic lecithin, is seen early in the third trimester; the desaturated form, dipalmitic lecithin (L), begins to appear at approximately 35 weeks' gestation and continues to increase throughout the remainder of the pregnancy. Sphingomyelin, a surfactant component without major surface activity properties, remains fairly constant during pregnancy. The L:S ratio measures the relationship between lecithin and sphingomyelin; if the increasing amount of lecithin over the relatively constant amount of sphingomyelin produces a ratio of 2:1 or more, fetal lung maturity is generally indicated, as long as the pregnancy is uncomplicated and the amniotic fluid sample is not contaminated with blood or meconium.20
A more reliable measure of fetal lung maturity is the "lung profile," in which the concentrations of the several lung surface lipids (i.e., PG, PI, PS, and PE) are measured in addition to lecithin and sphingomyelin. Next to lecithin, PG is the second major constituent of surfactant and is believed to aid in maintaining alveolar stability. PG appears in amniotic fluid at about 36 weeks' gestation and indicates secretion of mature surfactant. It has been found that, if PG is present in amniotic fluid, RDS in the newborn will not occur. PI is found in amniotic fluid before the appearance of PG and indicates immature fetal lungs. PI has a peak concentration at approximately 5 weeks before term and decreases thereafter. Measurement of PG and PI are most useful because they are unaffected by the presence of blood and meconium, although PG is affected by dilution of the specimen with water and by variations in test performance techniques. In addition to their usefulness in evaluating bloody or meconium-stained samples of amniotic fluid, lung profiles aid in determining lung maturity in fetuses of diabetic mothers. In maternal diabetes, the L:S ratio may indicate fetal lung maturity even though PG is not present. If the infant were delivered, RDS would be likely to occur. Measurement of PG in such situations aids in determining whether delivery should be attempted.21,22 The significance of measures of PS and PE in lung profiles has not yet been determined.
A bedside test to estimate fetal lung maturity can be performed when immediate results are needed. The shake test is based on the ability of surfactant to form stable foam, even in the presence of alcohol, which impairs foaming of most other biologic compounds. In this test, equal amounts of 95 percent ethanol and amniotic fluid are shaken together vigorously for 15 seconds and then allowed to stand undisturbed for 15 minutes. If a complete ring of bubbles is present at the meniscus, the test result is reported as positive, which indicates that sufficient lecithin is available for fetal lung maturity.23 The sample of amniotic fluid for the shake test can also be diluted with saline to various concentrations to estimate fetal lung maturity more accurately. The test result should be positive even when amniotic fluid has been diluted with two parts of saline.24
Other tests of amniotic fluid for fetal maturity include creatinine and bilirubin determinations and examination of fetal cells for type and lipid content. Creatinine appears in increased amounts in amniotic fluid at about the 36th week of gestation because of urinary excretion by the fetal kidneys and increased fetal muscle mass. A creatinine concentration of greater than 2.0 mg/dL indicates a fetal age of at least 36 to 37 weeks.25,26 As noted previously, bilirubin levels decline throughout the last several weeks of pregnancy. Thus, a bilirubin level of less than 0.025 mg/dL at term is considered an indication of fetal maturity. Note, however, that bilirubin levels cannot be used to assess fetal maturity in isoimmunized mothers, because levels will be elevated as a result of hemolytic disease involving the fetus.
During the second and third trimesters, fetal epithelial cells are shed into the amniotic fluid. As the fetus matures, the percentage of cells containing lipids increases. The test is performed by staining the cells with Nile blue stain. Cells containing lipid appear orange.27 Although only 1 percent of the cells contain lipid at 34 weeks' gestation, 10 to 50 percent contain lipid at 38 to 40 weeks.28 Fetal maturity may also be evaluated by examining the types of cells present. Whereas basal cells are present until about 32 weeks' gestation, cornified cells appear at 36 weeks and are the predominant cell type after 38 weeks.29
