Haptoglobin, an 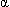 2-globulin produced in the liver, binds free hemoglobin released by the hemolysis of red blood cells in the bloodstream. Most red blood cells are normally removed in the reticuloendothelial system (e.g., liver, spleen) by a process known as extravascular destruction. Approximately 10 percent of red blood cells are, however, broken down in the circulation (intravascular destruction). This percentage may increase in situations caused by excessive red blood cell hemolysis (e.g., transfusion reaction, hemolytic anemia).
2-globulin produced in the liver, binds free hemoglobin released by the hemolysis of red blood cells in the bloodstream. Most red blood cells are normally removed in the reticuloendothelial system (e.g., liver, spleen) by a process known as extravascular destruction. Approximately 10 percent of red blood cells are, however, broken down in the circulation (intravascular destruction). This percentage may increase in situations caused by excessive red blood cell hemolysis (e.g., transfusion reaction, hemolytic anemia).
The free hemoglobin released from intravascular red blood cell destruction is unstable in plasma and dissociates into components (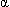 -
-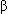 dimers) that are quickly bound to haptoglobin. Formation of the haptoglobin-hemoglobin complex prevents the renal excretion of plasma hemoglobin and stabilizes the heme-globin bond. The haptoglobin-hemoglobin complex is removed from the circulation by the liver.
dimers) that are quickly bound to haptoglobin. Formation of the haptoglobin-hemoglobin complex prevents the renal excretion of plasma hemoglobin and stabilizes the heme-globin bond. The haptoglobin-hemoglobin complex is removed from the circulation by the liver.
There is a limit to the capacity of the haptoglobin-binding mechanism, and a sudden intravascular release of several grams of hemoglobin can exceed binding capacity. Furthermore, because haptoglobin itself is removed from the circulation as a haptoglobin-hemoglobin complex and is catabolized by the liver, a decrease in or absence of haptoglobin may be used to indicate increased intravascular red blood cell hemolysis.
Because haptoglobin is formed in the liver, chronic liver disease with impaired protein synthesis also may result in decreased haptoglobin levels. Although haptoglobin is absent in most newborns, congenital absence of haptoglobin (congenital ahaptoglobinemia) can occur in a very small percentage of the population.
If haptoglobin is deficient or its binding capacity overwhelmed, unbound hemoglobin dimers are free to be filtered by the renal glomerulus, after which they are reabsorbed by the renal tubules and converted into hemosiderin (a storage form of iron). If renal tubular uptake capacity is exceeded, either free hemoglobin or methemoglobin (a type of hemoglobin with iron in the ferric, instead of the ferrous, form) is excreted in the urine. Note that reabsorption of free hemoglobin may damage the renal tubules because of excessive deposition of hemosiderin.18
Elevated haptoglobin levels are seen in inflammatory diseases (e.g., ulcerative colitis, arthritis, pyelonephritis) and in disorders involving tissue destruction (e.g., malignancies, burns, acute myocardial infarction). Steroid therapy may also elevate haptoglobin levels. Elevated levels are not of major clinical significance except to indicate that additional testing may be necessary to determine the source of the elevation.