Population: Adults with chronic HCV infection.
Organizations
 AASLD 2020, AGA 2017, EASL 2020
AASLD 2020, AGA 2017, EASL 2020
Recommendations
Evaluation
–If antibody test positive, reflex to an HCV RNA or core antigen confirmation test. In immunocompromised or chronic dialysis patients, use HCV RNA as the first-line test. (EASL)
–If in initial testing anti-HCV antibody is positive but RNA or core antigen are negative, test HCV RNA 12 to 24 wk later to confirm clearance. (EASL)
–Initiate antiviral treatment for all adults with acute or chronic HCV infection, except those with short life expectancy that will not improve with HCV therapy, liver transplant, or other therapies. Few contraindications exist otherwise.
–Consider urgent treatment for patients with significant cirrhosis, extrahepatic manifestations, recurrence after liver transplant, patients at risk for rapid progression because of comorbidities such as HIV or DM, and in individuals at high risk of transmitting HCV.
–Include the following in initial visits:
• History:
 HCV exposure risk factors and timing of exposure.
HCV exposure risk factors and timing of exposure.
 Symptoms of advanced liver disease such as jaundice, ascites, variceal bleeding, fatigue, pruritus, confusion.
Symptoms of advanced liver disease such as jaundice, ascites, variceal bleeding, fatigue, pruritus, confusion.
 Extrahepatic manifestations.
Extrahepatic manifestations.
 Prior HCV treatment.
Prior HCV treatment.
 Other medical issues: diabetes, CVA, anemia, CKD, HIV, hepatitis B coinfection, depression, solid organ transplant recipient.
Other medical issues: diabetes, CVA, anemia, CKD, HIV, hepatitis B coinfection, depression, solid organ transplant recipient.
 Family history of cirrhosis, liver cancer, alcohol dependence.
Family history of cirrhosis, liver cancer, alcohol dependence.
 Social history of past and current alcohol use, current illicit drug use.
Social history of past and current alcohol use, current illicit drug use.
• Labs:
 HCV RNA quantitative PCR.
HCV RNA quantitative PCR.
 HCV genotype (unless done previously). Resistance testing is not required prior to first-line treatment.
HCV genotype (unless done previously). Resistance testing is not required prior to first-line treatment.
 CBC.
CBC.
 Serum: Creatinine, sodium, potassium, chloride, albumin, total protein, total bilirubin, ALT, AST, alkaline phosphatase, glucose, protime (INR).
Serum: Creatinine, sodium, potassium, chloride, albumin, total protein, total bilirubin, ALT, AST, alkaline phosphatase, glucose, protime (INR).
 Hepatitis A and B immune status.
Hepatitis A and B immune status.
 Resistance-associated variant testing in patients with HCV genotype 1a who are using grazoprevir/elbasvir or prior treatment with a direct-acting antiviral agent.
Resistance-associated variant testing in patients with HCV genotype 1a who are using grazoprevir/elbasvir or prior treatment with a direct-acting antiviral agent.
 Hepatic fibrosis severity testing, preferably noninvasively.
Hepatic fibrosis severity testing, preferably noninvasively.
 Hepatic ultrasound.
Hepatic ultrasound.
Management
–Use a simple regimen with a single direct antiviral agent (DAA) for treatment-naïve adults without cirrhosis or with compensated cirrhosis. Some regimens treat certain genotypes, and some are pangenotypic. Updated guidelines are available at www.HCVGuidelines.org.
–Conditions requiring a more complex treatment regimen include ESRD, HIV or HBsAg positive, pregnancy, known or suspected HCC, and history of liver transplant.
–Evaluate for drug-drug interactions (www.hep-druginteractions.org) prior to initiating therapy. Interactions exist between certain DAAs and HIV therapies, opiates, lipid-lowering drugs, antipsychotics, antiarrhythmics, antihypertensives, immunosuppressants, antiplatelet agents, anticoagulants, anticonvulsants, PPIs, and others.
–Interferon regimens are the only option for HCV infected or HIV/HCV infected with decompensated cirrhosis.
–There is very limited evidence for treating patients with mixed genotypes (ie, multiple genotypes of HCV infection concurrently). Consider using a pangenotypic regimen and if the optimal regimen or duration is unclear, consult a specialist.
–Treatment monitoring for 8, 12, and 16 wk regimens. (AGA)
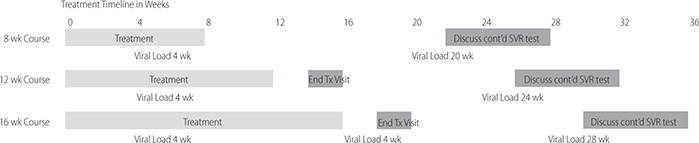
–Vaccinate patients who lack antibodies for hepatitis A and B viruses. If cirrhosis, give pneumococcal vaccine.
–Educate persons with current HCV infection on methods to reduce progression of liver disease (eg, alcohol abstinence) and avoid transmission to others.
–Insufficient evidence to recommend herbal therapy.
–Include the following in subsequent visits:
• For hepatic fibrosis stage 3 or 4:
 EGD evaluation for esophageal varices.
EGD evaluation for esophageal varices.
 Alpha fetoprotein.
Alpha fetoprotein.
 Hepatic ultrasound (or, if images inadequate, CT scan of abdomen with contrast).
Hepatic ultrasound (or, if images inadequate, CT scan of abdomen with contrast).
 Risk reduction/mitigation:
Risk reduction/mitigation:
 Hepatitis A and B vaccinations if not immune.
Hepatitis A and B vaccinations if not immune.
 Age-appropriate vaccinations and cancer screening.
Age-appropriate vaccinations and cancer screening.
 Counseling on alcohol abstinence.
Counseling on alcohol abstinence.
 Counseling on transmission/reinfection of HCV.
Counseling on transmission/reinfection of HCV.
 Management of comorbid conditions.
Management of comorbid conditions.
 Counseling on adherence and consequences of treatment failure if being treated.
Counseling on adherence and consequences of treatment failure if being treated.
–Reassess labs within 12 wk of starting treatment which include: CBC, INR, hepatic function panel (albumin, total and direct bilirubin, ALT/AST, alk phos), renal function (eGFR). (AGA)
–Refer patients with advanced cirrhosis, multiple treatment failures, coinfection with HIV or hepatitis B to hepatology clinic for proper management. (AGA)
–Declare sustained viral response if RNA or core antigen are undetectable 12–24 wk after the end of treatment.
–Long-term monitoring after successful treatment:
• Fibrosis score 0–2: no further monitoring; counsel on reinfection risk, HIV prevention.
• Fibrosis score 3–4: Ongoing HCC monitoring q 6 mo (hepatic ultrasound, AFP levels, LFTs, renal function, INR); yearly visits with hepatology.
Practice Pearl
• High-risk patients are defined as “history of injection drug use, transfusion or organ transplant before 1992, received clotting factors before 1987, history of long-term dialysis, HIV infection, persistently elevated liver enzymes, health care and public safety workers after needle sticks, sharps, or mucosal exposure to HCV-positive blood, and children born to HCV-positive women.”
Sources
–https://doi.org/10.1002/hep.31060
–https://doi.org/10.1053/j.gastro.2017.03.039
–https://doi.org/10.1016/j.jhep.2020.08.018
Population: Adults with acute HCV infection.
Organizations
 AASLD 2020, EASL 2020
AASLD 2020, EASL 2020
Recommendations
–Initiate HCV treatment without awaiting spontaneous resolution. Use the same regimens recommended for chronic HCV infection.
–Assess sustained viral response at 12 and 24 wk as late relapses have been reported.
Sources
–https://doi.org/10.1002/hep.31060
–https://doi.org/10.1016/j.jhep.2020.08.018
Population: Children with HCV infection.
Organizations
 AASLD 2020, EASL 2020
AASLD 2020, EASL 2020
Recommendations
–Treat patients >3-y-old if a DAA regimen is available for their genotype and age range. Start treatment as soon as possible if the child has cryoglobulinemia, rashes, and glomerulonephritis or advanced fibrosis.
–Avoid interferon-based treatment regimens in children and adolescents.
–Surveil for HCC and varices in children with cirrhosis.
–Therapeutic dose of acetaminophen, steroids, chemotherapy, and organ and bone marrow transplant are not contraindicated in children with chronic hepatitis C.
–HCV is not transmitted through casual contact, so HCV-infected children do not pose a risk to other children. They can participate in school, sports, athletics, and regular childhood activities without restriction.
–Use universal precautions at school and in the home. Advise family members not to share toothbrushes, razors, nail clippers, and to use gloves and dilute bleach to clean up blood.
Sources