C.
Explanation
Uridine triacetate (Vistogard) is a pyrimidine analog that was FDA-approved in 2015 for the emergency treatment of adult and pediatric patients following 5-fluorouracil (5-FU) or capecitabine overdose regardless of the presence of symptoms. It is also approved for patients who exhibit early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and /or early-onset, unusually severe adverse reactions within 96 hours following the end of 5-FU or capecitabine administration. The adult dosing is 10 g (1 packet) orally every 6 hours for 20 doses. The reaction below shows conversion of uridine triacetate: 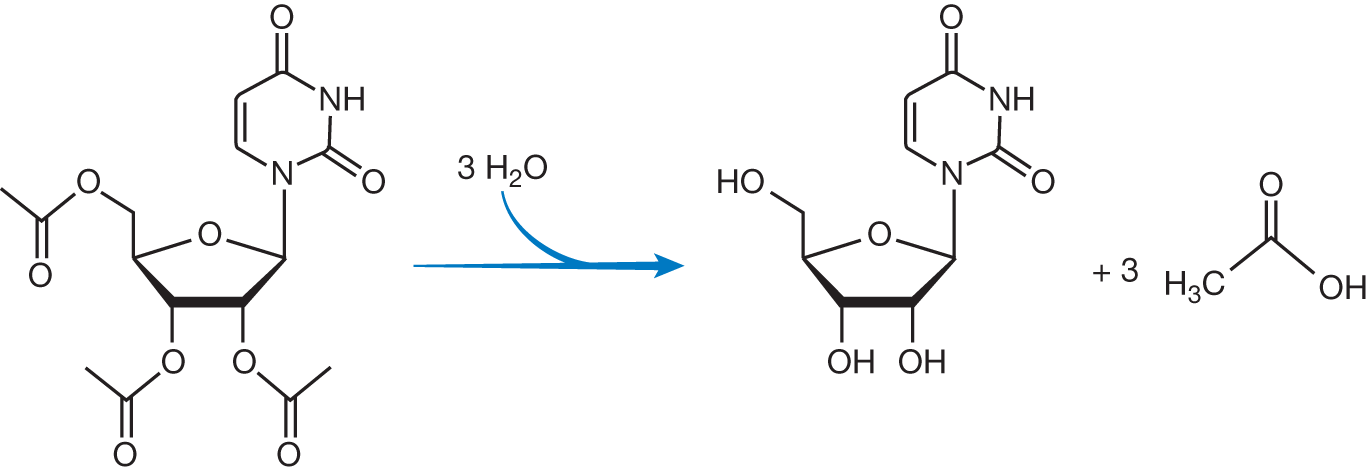
Leucovorin is used to prevent the toxicities of high-dose methotrexate. Dexrazoxane is used for both prevention of cardiotoxicity from anthracycline-based chemotherapy and for prevention of tissue injury after anthracycline extravasation. Glucarpidase is a recombinant bacterial enzyme that is a rescue agent for patients with toxic plasma methotrexate concentrations in patients with delayed methotrexate excretion and impaired renal function.