Platinum Compounds.
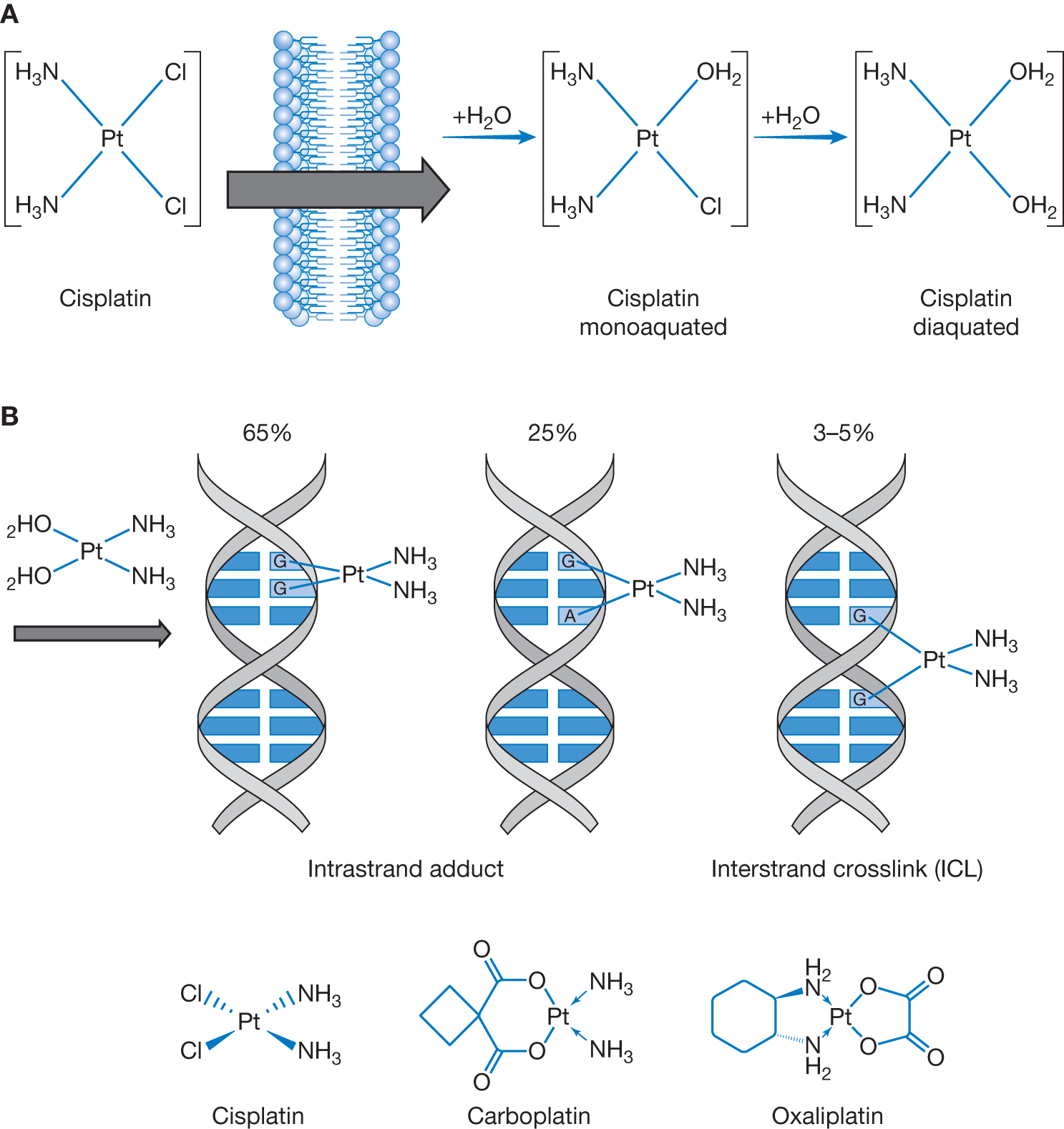
| Platinum agents are grouped with alkylators in that they are alkylating-like, resulting in intrastrand and interstrand crosslinking. They accomplish this crosslinking, however, without addition of alkyl groups. In 1965, the antitumor activity of Cisplatin was first discovered during a study on the effect of electric current on bacterial growth, and in early 1970s was later developed as a modern chemotherapy. Cisplatin-based chemotherapy is responsible for the high cure rate of germ cell tumors, high response and remission rates of ovarian cancer and small cell lung cancer, and stabilizing various cancers, including non-small cell lung cancer, and head and neck, bladder, stomach, anal, and cervical cancer. The dose of cisplatin completely varies from one chemotherapy regimen to another one. When cisplatin is used concurrently with radiation therapy, it acts as a potent radiation sensitizer. The observed adverse clinical effects of cisplatin in nephrotoxicity and ototoxicity are the result of depleted glutathione with resultant increase in reactive oxidative species. Carboplatin has reduced side-effects compared with cisplatin. The Calvert formula is used to calculate the dose of carboplatin, which considers the creatinine clearance and the desired area under curve (represents the total drug exposure over time). Calvert formula: total carboplatin dose (mg) = (target AUC) × (glomerular filtration rate [GFR] + 25). With almost similar efficacy, carboplatin can substitute cisplatin for the treatment of most solid tumors, except bladder cancer. Oxaliplatin is used for treatment of many gastrointestinal malignancies in combination with 5-FU and leucovorin (e.g., FOLFOX). Platinum is eliminated via kidney and requires dose adjustment for renal dysfunction. Platinum does NOT require dose adjustment for hepatic dysfunction. Platinum should be administered after taxanes to decrease myelosuppression and enhance efficacy. Adverse events of interest for each platinum compound:
|