Mechanism of action of DNA alkylation by nitrogen mustards.
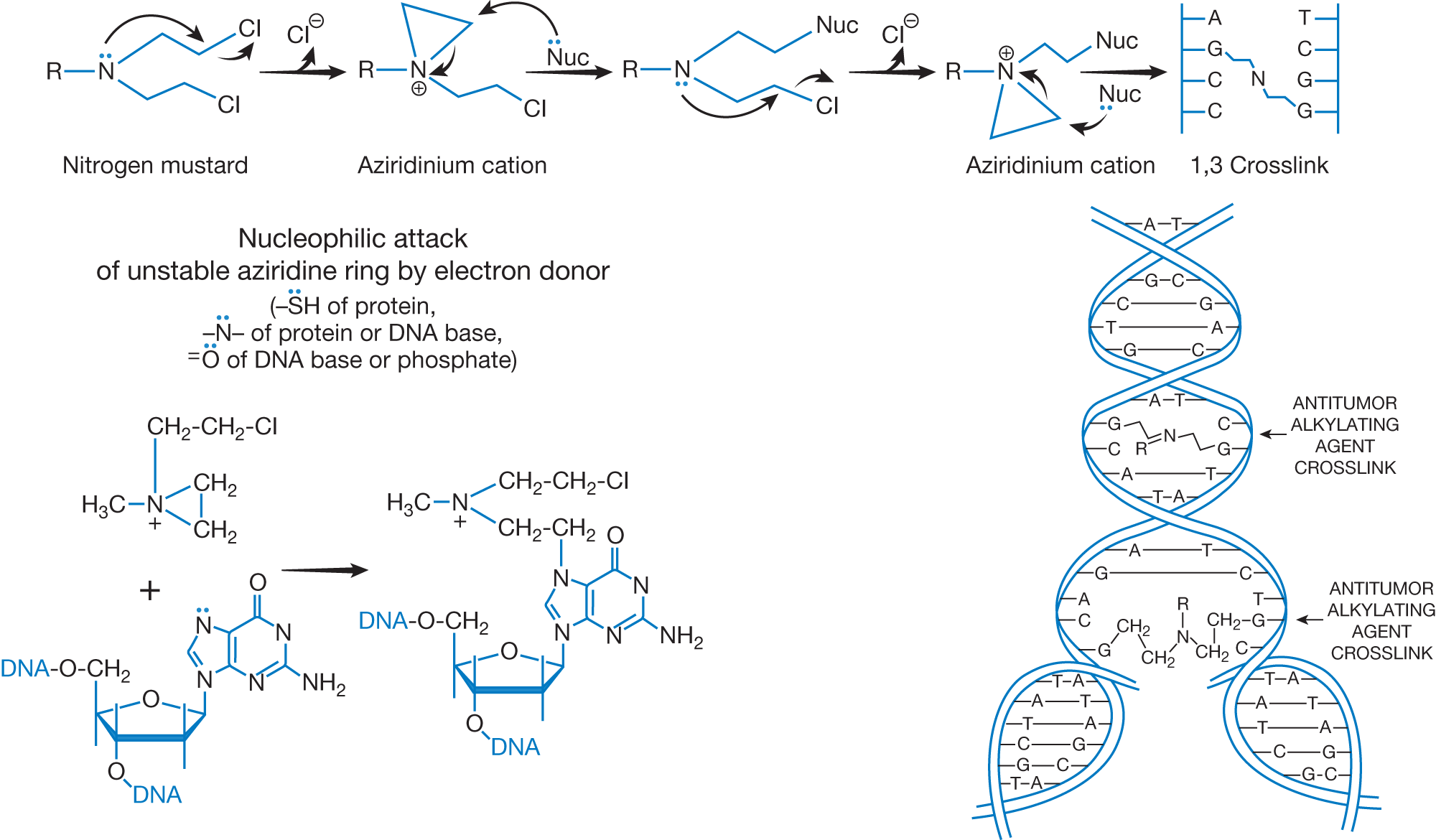
In a nitrogen mustard, the nitrogen atom displaces the chloride to result in a strained, three-membered intermediate cation called aziridinium. This ion easily forms covalent bonds with nucleophiles, such as nitrogens of DNA (e.g., N7 position of guanine) or proteins, oxygen atoms of DNA, or sulfur atoms of proteins. The second chemical transformation of the nitrogen mustard provides another aziridinium ion, which upon reaction with another DNA nucleophile, forms a DNA crosslink.