DESCRIPTION 
Hypertrophic cardiomyopathy (HCM) is a heritable disease caused by abnormal sarcomeric proteins resulting in LV septal hypertrophy, typically with LV outflow tract (LVOT) obstruction, resulting in various clinical symptoms including syncope, heart failure, angina, atrial and ventricular arrhythmias, and sudden cardiac death.
EPIDEMIOLOGY 
- Estimated prevalence 0.2% among the general adult population in the U.S.
- Prevalence: Male = Female
- HCM is thought to be underdiagnosed in women, minorities, and underserved populations.
- HCM is the most common cause of sudden cardiac death in young people, including young competitive athletes (accounting for 36% of cases).
- 70% of HCM patients have an LVOT gradient of
 30 mm Hg at rest or with exertion, while 30% have nonobstructive HCM.
30 mm Hg at rest or with exertion, while 30% have nonobstructive HCM.
RISK FACTORS 
Family history of HCM
Genetics 
- >400 mutations (mostly missense) in 11 genes encoding various sarcomeric proteins have been described. The predominant mutations involve
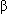 -myosin heavy chain, myosin binding protein C, and troponin-T. Other affected myofilaments include myosin light chains, titin, troponin-I,
-myosin heavy chain, myosin binding protein C, and troponin-T. Other affected myofilaments include myosin light chains, titin, troponin-I, 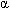 -actin, and
-actin, and 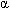 -tropomyosin.
-tropomyosin. - Autosomal-dominant mode of inheritance.
- Phenotypic expression (ie, LVH) is also dependent upon modifier genes and environmental factors.
- Many patients have no affected relatives.
- Increased risk for atrial fibrillation with
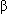 -myosin heavy chain Arg663 His mutation. Potentially higher risk for sudden death with other
-myosin heavy chain Arg663 His mutation. Potentially higher risk for sudden death with other 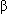 -myosin heavy chain mutations.
-myosin heavy chain mutations.
Pregnancy Considerations 
Pregnancy is not contraindicated; overall pregnancy-related serious morbidity/mortality is low (1–2% of all pregnancies); vaginal delivery is tolerated; use caution with epidural anesthesia as it can cause vasodilation and exacerbate LVOT gradient.
PATHOPHYSIOLOGY 
- Asymmetric LV hypertrophy causes LVOT obstruction. In most cases, systolic anterior motion (SAM) of the mitral valve (with resultant MR) contributes to LVOT obstruction; however, an intracavitary gradient may exist without SAM.
- The pressure gradient and LVOT obstruction are dynamic, exacerbated by factors such as exertion, orthostatic changes, hypovolemia, Valsalva, PVCs, tachyarrhythmias, and vasodilator therapy.
- LVOT obstruction can lead to syncope, heart failure due to elevated pulmonary venous pressures, ischemia due to increased LV wall stress and oxygen demand, atrial fibrillation due to MR and LA enlargement.
- Sudden death can occur even in the absence of LVOT obstruction.
ETIOLOGY 
Genetic mutations result in production of abnormal cardiac sarcomeric proteins. Phenotypic expression (ie, LVH) typically occurs during adolescence, but can occur at any time, even in the elderly.
Outline
History 
- HCM may be asymptomatic.
- Syncope and/or dizziness: The most common symptoms; occur at rest or with exertion/postural changes (due to LVOT obstruction or ventricular arrhythmia)
- Angina (due to supply/demand mismatch, or "tunneling" of epicardial coronary arteries in the myocardium)
- Heart failure symptoms, including dyspnea (at rest or with exertion), fatigue, orthopnea, paroxysmal nocturnal dyspnea (due to LV diastolic dysfunction and, in some cases, systolic dysfunction)
- Palpitations if atrial fibrillation is present
- Sudden death, typically with exertion but may also occur at rest
- Inquire about family history of HCM, syncope, and sudden death. Family history is helpful for diagnosis in cases when echo has borderline HCM features.
Physical Exam 
- Physical exam may be normal if there is no LV cavitary pressure gradient or LV dysfunction.
- Diffuse or laterally displaced PMI; palpable double or triple LV apical impulse.
- Bifid carotid pulse (rapid upstroke followed by 2nd peak due to hyperdynamic LV).
- S2 paradoxically split due to prolonged ejection time with severe LVOT gradient.
- S4
- LVOT obstruction murmur: Harsh, crescendo-decrescendo murmur at left sternal border; decreases with squatting and handgrip; increases upon standing and Valsalva.
- Mitral regurgitation murmur.
DIAGNOSTIC TESTS & INTERPRETATION
Lab 
- EKG: Abnormal in 90–95% of cases. May have any of the following: LVH voltage; ST-T waves changes including deep T wave inversions in the precordial leads (common in apical HCM among Japanese people, called Yamaguchi’s HCM); left atrial enlargement; Q waves; diminished R waves in the lateral precordial leads; atrial fibrillation; PVCs.
- Plasma brain natriuretic peptide (BNP): Not useful.
Imaging 
- 2D echo is the cornerstone of diagnosis. Findings include asymmetrical septal hypertrophy (classically septal, but other morphologies exist) with end-diastolic wall thickness typically >15 mm; small LV cavity with septal immobility; SAM of the mitral valve; MR and potentially MVP; LA enlargement; premature AV closure; LVOT pressure gradient (
 30 mm Hg at rest and
30 mm Hg at rest and  50 mm Hg with exercise or pharmacological provocation); impaired LV relaxation and filling by Doppler interrogation; potentially LV systolic dysfunction.
50 mm Hg with exercise or pharmacological provocation); impaired LV relaxation and filling by Doppler interrogation; potentially LV systolic dysfunction. - Cardiac MRI:
- May detect LV thickening not noted on echo (particularly of the LV free wall). MRI is also more sensitive than echo for detection of MR, SAM, and abnormal papillary muscles
- Myocardial scar may be detected as delayed hyperenhancement with gadolinium.
Diagnostic Procedures/Surgery 
- Holter monitor: May demonstrate paroxysmal atrial fibrillation or NSVT (helpful for risk stratification to determine whether ICD is indicated).
- Genetic testing:
- A test for mutations in the 8 myofilament-encoding genes most commonly associated with HCM is available (visit www.hpcgg.org/lmm for information).
- Not required for diagnosis, but may help with prognosis in the future as the test is refined.
- Cardiac catheterization: Typically not indicated unless planning for myectomy or performing alcohol septal ablation.
- Hemodynamic findings include subaortic or midventricular pressure gradient on catheter pullback; elevated RV and LV end diastolic pressures; elevated PCWP; Brockenbrough-Braunwald-Morrow sign (marked LVOT pressure gradient on post-extra-systolic beat, leading to a diminished pulse pressure due to reduced stroke volume).
- Screening of family members:
- Screening of 1st-degree relatives is recommended.
- Evaluation should include history, physical, EKG, and 2D-echo.
- Repeat evaluations every 12–18 mo between ages 12 and 21 yr (since phenotypic expression of HCM usually occurs during adolescence). After age 21, evaluate about every 5 yr.
Pathological Findings 
- Gross: Increased LV weight and wall thickness (often asymmetrical); a distinct subset have anomalous insertion of the anterolateral papillary muscle directly into the anterior MV leaflet.
- Histology: Misshapen, hypertrophied cardiomyocytes; disorganized array of myocytes and myofibrils; disorganized and expanded LV collagen matrix; intimal and medial thickening of the intramural coronary arteries, with luminal stenosis; replacement fibrosis—often in anatomical proximity to abnormal intramural coronary arteries. Histological abnormalities may be present in both hypertrophied and non-hypertrophied regions of myocardium.
DIFFERENTIAL DIAGNOSIS 
- Other causes of LV hypertrophy: Athlete’s heart; HTN; aortic stenosis; amyloidosis; sarcoidosis; hemochromatosis; Fabry disease; Pompe disease; Danon disease; Noonan syndrome.
- Athlete’s heart: Some echo findings may help distinguish HCM from athlete’s heart: HCM usually has unusual, asymmetric patterns of hypertrophy; substantial septal wall thickening (>15 mm); left atrial enlargement; abnormal LV relaxation and filling; LV end-diastolic diameter <45 mm (athlete’s heart may even have a dilated LV chamber >55 mm).
Outline
In general, treatment involves management of symptoms of syncope/presyncope related to LVOT gradient; heart failure related to LV diastolic and systolic dysfunction; angina related to supply/demand mismatch; atrial fibrillation which may result from left atrial enlargement; and prevention of sudden cardiac death.
SURGERY 
- Surgical myotomy-myectomy:
- For symptoms related to LVOT gradient, refractory to medical management
- Introduced in the 1960s; well established and refined procedure with long-term follow-up data.
- <1–2% operative mortality, and <3% operative morbidity at experienced centers. Adverse outcomes include High-grade AV block requiring pacemaker (1–2%); VSD (rare); aortic insufficiency.
- Success rate (reduced LVOT gradient and relief of symptoms):
 90%. Long-term studies show continued relief up to 25 yr after procedure in 85% of cases.
90%. Long-term studies show continued relief up to 25 yr after procedure in 85% of cases. - Myectomy also improves long-term survival compared to patients with LVOT obstruction who do not undergo myectomy.
- Percutaneous alcohol septal ablation:
- Alternative invasive approach for refractory symptoms related to LVOT obstruction in patients who would not tolerate surgery or are opposed to surgery.
- Percutaneous catheter-directed injection of 96–100% alcohol into septal perforating branch(es) of LAD, resulting in "controlled" infarct with eventual thinning of the septum to reduce outflow tract obstruction.
- Success rate: 70–80%.
- Procedural/long term complications: High-grade AV block (5–40%) requiring pacemaker (5–10%); VSD; scar-related ventricular arrhythmia.
- Implantable cardioverter-defibrillator:
- Indications include any 1 of the following high-risk features: Previous cardiac arrest; sustained VT or frequent NSVT on Holter monitor; recurrent syncope or near-syncope (especially if exertion-induced); decrease or lack of expected increase in blood pressure during exercise test; family history of sudden death or HCM-related syncope; severe LV hypertrophy (>30 mm); resting LV intra-cavitary gradient
 30 mm Hg or dynamic gradient
30 mm Hg or dynamic gradient  50 mm Hg.
50 mm Hg.
- Dual chamber pacemaker: Mixed evidence regarding the beneficial effect of dual chamber pacing on symptoms of LVOT obstruction
- Direct-current cardioversion: May be attempted for atrial fibrillation
- Heart transplantation: May be considered for end-stage heart failure
IN-PATIENT CONSIDERATIONS
Admission Criteria 
Syncope; decompensated heart failure; atrial fibrillation without adequate rate control
IV Fluids 
Hydration may reduce LVOT pressure gradient.
Outline
FOLLOW-UP RECOMMENDATIONS
Patient Monitoring 
Routine surveillance including history, physical, EKG, and echo every 12–18 mo is recommended.
PATIENT EDUCATION 
- HCM patients <30 are advised not to participate in competitive sports.
- Maintain adequate volume intake to avoid dehydration, which could exacerbate LVOT obstruction. Patients with heart failure in addition to LVOT obstruction need vigilant weight monitoring to avoid both volume depletion and volume overload.
PROGNOSIS 
- Annual mortality rate 1%.
- Having just 1 high-risk feature (as noted above under indications for ICD) increases risk for sudden cardiac death.
- There may be a reduction in chance of sudden cardiac death with age.
COMPLICATIONS 
Syncope; atrial and ventricular arrhythmias; heart failure; sudden death
Outline
 30 mm Hg at rest or with exertion, while 30% have nonobstructive HCM.
30 mm Hg at rest or with exertion, while 30% have nonobstructive HCM.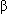 -myosin heavy chain, myosin binding protein C, and troponin-T. Other affected myofilaments include myosin light chains, titin, troponin-I,
-myosin heavy chain, myosin binding protein C, and troponin-T. Other affected myofilaments include myosin light chains, titin, troponin-I, 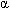 -actin, and
-actin, and 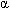 -tropomyosin.
-tropomyosin.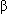 -myosin heavy chain Arg663 His mutation. Potentially higher risk for sudden death with other
-myosin heavy chain Arg663 His mutation. Potentially higher risk for sudden death with other 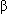 -myosin heavy chain mutations.
-myosin heavy chain mutations.