DESCRIPTION 
- Cardiomyopathy:
- Cardiomyopathy secondary to chemotherapy is most commonly associated with doxorubicin (Adriamycin), but also has been reported with other anthracycline agents such as daunorubicin and epirubicin, the anthraquinone agent mitoxantrone, trastuzumab (Herceptin), sunitinib, and bevacizumab (Avastin).
- Myocardial ischemia:
- Dysrhythmia:
- Arrhythmias may occur in the context of anthracycline-induced cardiomyopathy, but ventricular and supraventricular tachycardias also have been described with interferon and rituximab. Taxol has been associated with ventricular tachycardia, sinus bradycardia, and heart block. Thalidomide has been associated with bradycardia. Arsenic trioxide and dasatinib can cause QT prolongation.
- Other:
- Cisplatin, bevacizumab, and sorafenib commonly cause HTN. Hemorrhagic myopericarditis has been associated with high-dose cyclophosphamide. Imatinib (Gleevec) has been associated with pericardial effusion, heart failure, and edema/fluid retention. Bevacizumab has been associated with arterial thrombotic events including MI.
EPIDEMIOLOGY 
- Predominant age: Rates are higher at extremes of patient age, in elderly patients >70 yr of age, and in pediatric patients <15 yr of age.
- Predominant sex: Male = Female
Incidence 
The overall incidence of anthracycline-induced cardiomyopathy in the U.S. is ~2.2%.
RISK FACTORS 
- In addition to age, the following risk factors increase the incidence of anthracycline-induced cardiomyopathy by as much as 8–10-fold:
- Increased cumulative dose:
- The incidence of cardiomyopathy with doxorubicin administration at doses <400 mg/m2 is ~3–5%.
- The incidence has been reported to be as high as 26% at 550 mg/m2, and escalates sharply at higher doses (18–48% incidence at doses >700 mg/m2).
- Therefore, 550 mg/m2 is considered to be the upper limit for doxorubicin administration in most clinical scenarios.
- Rapid schedule of administration:
- Less cardiotoxicity has been reported when doxorubicin infusion is prolonged over 48–96 hr than when compared with standard 1–2-hr infusions.
- Other underlying heart disease:
- Anthracycline-induced cardiomyopathy is more common in the presence of preexisting heart diseases, particularly those that increase LV wall stress.
- These include hypertensive heart disease, hypertensive obstructive cardiomyopathy, aortic stenosis, or other forms of underlying cardiomyopathy.
- Concomitant therapy for malignancy:
- Radiation therapy through a portal that includes the heart (such as mediastinum, breast, or lung) has been shown to substantially increase the risk of developing anthracycline-induced cardiomyopathy.
- Other chemotherapeutic agents, such as concomitant administration of cyclophosphamide, trastuzumab, or mitomycin C chemotherapy, may increase the risk of developing cardiomyopathy, although available data are somewhat variable.
Genetics 
Unknown.
GENERAL PREVENTION 
- Care should be taken in dosing strategies for patients with baseline LV dysfunction and for patients with other risk factors for cardiotoxicity.
- Patients with severely reduced baseline LV ejection fraction (LVEF <30%) should not receive doxorubicin chemotherapy.
- Dexrazoxane, an iron-chelating agent that reduces free radical production, has been approved for use in patients with metastatic breast cancer receiving >300 mg/m2 of doxorubicin.
- A small randomized controlled trial showed that carvedilol prevented systolic and diastolic dysfunction in patients treated with anthracycline chemotherapy.
- Elevations in troponin I (TnI) >0.5 ng/mL immediately after or soon after administration of high-dose chemotherapy, has been associated with decline of LVEF up to 30% just 7 mo after chemotherapy. A randomized controlled trial showed that enalapril administered to patients with elevated TnI soon after high-dose chemotherapy reduced the incidence of LV systolic function.
PATHOPHYSIOLOGY 
Anthracycline-induced myocardial necrosis is due to direct membrane lipid damage and local free radical production. Vasospasm can occur with 5-fluorouracil and other agents.
ETIOLOGY 
Chemotherapeutic agent-induced myocardial and vascular inflammation, disease, and damage
COMMONLY ASSOCIATED CONDITIONS 
Anemia
Outline
History 
- Heart failure due to anthracycline-induced cardiotoxicity is clinically indistinguishable from other etiologies of biventricular heart failure.
- Symptoms:
- Fatigue
- Exercise intolerance
- Dyspnea
- Paroxysmal nocturnal dyspnea
- Orthopnea
- Edema
- Palpitations, syncope, or pre-syncope
Physical Exam 
- Resting tachycardia
- Jugular venous distention
- S3 (3rd heart sound) gallop
- Pulmonary congestion
- Peripheral edema
DIAGNOSTIC TESTS & INTERPRETATION
Lab 
CBC may show anemia.
Initial lab tests
EKG abnormalities are nonspecific and may be transient:
- Sinus tachycardia
- ST- or T-wave changes
- Premature ventricular contractions or other dysrhythmias
- Decreased voltage
- QT-interval prolongation
Imaging 
- CXR:
- Cardiac enlargement
- Pulmonary vascular plethora
- Pleural effusions
- Radionuclide ventriculography
- Echo: Has the advantage over ventriculography in that diastolic dysfunction, valvular dysfunction, and pericardial disease can also be detected:
- Biventricular enlargement
- Abnormal systolic function: Reduced LVEF and fractional shortening, most commonly without segmental wall motion abnormality (global hypokinesis).
- Doppler evidence of abnormal diastolic function.
- Stress imaging to evaluate LV response to exercise may add sensitivity to either radionuclide or echo imaging.
Diagnostic Procedures/Surgery 
- Endomyocardial biopsy:
- Best method for absolute quantification of cardiac damage and may be used to guide decisions about subsequent anthracycline dosing.
- Cardiac muscle involvement may be patchy, so multiple RV specimens should be obtained when possible.
- Pathology reveals swelling of sarcoplasmic reticulum and mitochondria, myofibrillar dropout, vacuole formation, and frank myocyte necrosis.
- Pathologic severity is graded on a scale of 0–3, with biopsy grade >1.5 considered a contraindication to continuing anthracycline therapy.
- Clinical manifestations:
- Acute toxicity occurs either during or immediately after treatment (even a single cycle of drug):
- Acute toxicity is rare (<1%) and is most commonly manifested as a transient electrocardiographic abnormality or dysrhythmia
- However, it can present as myocarditis, pericarditis, fulminant cardiac failure, or even sudden death.
- Early-onset chronic progressive form occurs in 1–2% of patients, either during therapy or within the 1st year of therapy
- Late-onset chronic progressive cardiotoxicity occurs in 1.6–5% of patients and presents as dilated cardiomyopathy. This may not become clinically evident until 10–20 yr after the 1st dose of treatment.
- Asymptomatic reduction in LV systolic function is the most common manifestation of chronic anthracycline-induced cardiomyopathy:
- The criteria for asymptomatic cardiomyopathy are an absolute decrease in LVEF of >10% or a decline in LVEF to <50% when previously normal.
- Less commonly, anthracycline-induced cardiomyopathy may present as overt clinical CHF.
Pathological Findings 
Cardiac necrosis
DIFFERENTIAL DIAGNOSIS 
- Underlying cardiovascular disease, which may have been previously subclinical
- Other cardiac complications of malignancy:
- Metastatic or primary tumor involvement of the pericardium or myocardium
- Obstruction of venous inflow [SVC or IVC (superior or inferior vena cava) syndrome].
- Pulmonary embolism with chronic pulmonary emboli leading to pulmonary HTN and RV failure.
- Radiation-induced pericardial, myocardial, or coronary artery disease
- Dysrhythmia secondary to other drugs or metabolic abnormalities
Outline
ADDITIONAL TREATMENT
General Measures 
- Unless acute decompensated heart failure occurs, patients can be managed as outpatients.
- Treatment for documented anthracycline-induced cardiomyopathy:
- Anthracycline administration should be discontinued.
- Baseline screening should include:
- History and physical exam
- EKG
- CXR
- Assessment of LV function by echo or radionuclide ventriculography
Referral 
Symptoms of heart failure, signs of heart failure or LVEF < normal should occasion a cardiology consultation and follow-up.
Additional Therapies 
Coenzyme Q10 has been used to protect the heart from toxicity, but there are no convincing studies to support it.
SURGERY 
Selected patients who no longer have signs of malignancy may be candidates for cardiac transplantation, but the risks of immunosuppression-induced recurrence of malignancy must be weighed against the benefits of transplantation.
IN-PATIENT CONSIDERATIONS
Initial Stabilization 
Appropriate treatment for heart failure, arrhythmias, or myocardial ischemia
Admission Criteria 
Standard admission for acute heart failure, ischemia, or arrhythmia
Discharge Criteria 
Resolution of symptoms of heart failure, ischemia, or arrhythmia
Outline
FOLLOW-UP RECOMMENDATIONS
Patient Monitoring 
- Guidelines for the frequency of follow-up are as follows:
- Patients with normal baseline LVEF (>50%):
- Repeat assessment of LV function after 200–300 mg/m2 and again after 450 mg/m2 or after 400 mg/m2 in patients with other risk factors for cardiomyopathy.
- Repeat assessment before every dose after 400–450 mg/m2
- Discontinue doxorubicin when LVEF falls by >10% or absolute LVEF <50%.
- Patients with moderately reduced baseline LVEF (30–50%):
- Repeat assessment of LVEF before every dose.
- Discontinue doxorubicin when LVEF falls by >10% or absolute LVEF <30%.
DIET 
Low salt
PATIENT EDUCATION 
Continue cardiac medications and follow-up until problem resolved.
PROGNOSIS 
- Prognosis is poorer in patients who develop overt CHF, with reported mortality rates as high as 43%.
- Outcomes in patients with CHF are improved with conventional medical therapy, including
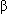 -blockers and ACE inhibitors.
-blockers and ACE inhibitors. - Reversal of even severe cardiac dysfunction has been reported.
COMPLICATIONS 
Cardiac death, persistent heart failure
Outline

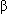 -blockers and ACE inhibitors.
-blockers and ACE inhibitors.