Digoxin is used most often during the perioperative period for the management of supraventricular tachydysrhythmias. The use of digoxin to treat acute decreases in left ventricular contractility is uncommon because of the availability of more potent and less toxic drugs. Digoxin may be selected only for treatment of symptoms that persist after administration of angiotensin-converting enzyme inhibitors or
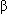 -adrenergic antagonists.
-adrenergic antagonists.IV administration of propranolol or esmolol combined with digoxin may provide more rapid control of supraventricular tachydysrhythmias and minimize the likelihood of toxicity by permitting decreases in the dose of both classes of drugs.
Direct current cardioversion in the presence of digoxin may be hazardous because of increased risk for developing cardiac dysrhythmias, including ventricular fibrillation.
In approximately 30% of patients with Wolff-Parkinson-White syndrome, digitalis decreases refractoriness in the accessory conduction pathway to the point that rapid atrial impulses can cause ventricular fibrillation.
Digoxin may be harmful in patients with hypertrophic subaortic stenosis because increased myocardial contractility intensifies the resistance to ventricular ejection.
Pharmacokinetics. Digoxin is eliminated almost entirely by renal excretion, with a half-life of 1 to 2 days. The half-life is inversely proportional to glomerular filtration rate and thus increases with age or renal disease.
Mechanism of Action
Cardiac glycosides selectively and reversibly inhibit the sodium-potassium adenosine triphosphatase (ATPase) ion transport system (sodium pump) located in the sarcolemma (cell wall) of cardiac cells.
In addition to positive inotropic effects, cardiac glycosides enhance parasympathetic nervous system activity, leading to delayed conduction of cardiac impulses through the atrioventricular node and decreases in heart rate.
The electrophysiologic effects of therapeutic plasma concentrations of cardiac glycosides manifest on the electrocardiogram (ECG) as (a) prolonged P-R intervals due to delayed conduction of cardiac impulses through the atrioventricular node, (b) shortened QTc intervals because of more rapid ventricular repolarization, (c) ST segment depression (scaphoid or scooped-out) due to a decreased slope of phase 3 depolarization of cardiac action potentials, and (d) diminished amplitude or inversion of T waves. The P-R interval is rarely prolonged to longer than 0.25 second.
When digitalis is discontinued, the changes on the ECG disappear in several weeks.
Toxicity. Cardiac glycosides have a narrow therapeutic range (estimated that approximately 20% of patients who are being treated with cardiac glycosides experience some form of digitalis toxicity). The most frequent cause of digitalis toxicity in the absence of renal dysfunction is the concurrent administration of diuretics that cause potassium depletion. During anesthesia, hyperventilation of the patient’s lungs can decrease the serum potassium concentration an average of 0.5 mEq/L for every 10 mm Hg decrease in Paco2. Hypokalemia probably increases myocardial binding of cardiac glycosides, resulting in an excess drug effect.
Diagnosis. Digoxin is often administered in situations where digitalis toxicity is difficult to distinguish from the effects of the cardiac disease.
Determination of the plasma digoxin concentration may be used to indicate the likely presence of digitalis toxicity. A plasma digoxin concentration of less than 0.5 ng/mL eliminates the possibility of digitalis toxicity. Anorexia, nausea, and vomiting are early manifestations of digitalis toxicity. These symptoms, when present preoperatively in patients receiving cardiac glycosides, should arouse suspicion of digitalis toxicity.
There are no unequivocal features on the ECG that confirm the presence of digitalis toxicity. Nevertheless, toxic plasma concentrations of digitalis typically cause atrial or ventricular cardiac dysrhythmias (increased automaticity) and delayed conduction of cardiac impulses through the atrioventricular node (prolonged P-R interval on the ECG), culminating in incomplete to complete heart block.
Treatment of digitalis toxicity includes (a) correction of predisposing causes (hypokalemia, hypomagnesemia, arterial hypoxemia), (b) administration of drugs (phenytoin, lidocaine, atropine) to treat cardiac dysrhythmias, and (c) insertion of a temporary artificial transvenous cardiac pacemaker if complete heart block is present.
Supplemental potassium decreases the binding of digitalis to cardiac muscle and directly antagonizes the cardiotoxic effects of cardiac glycosides. Serum potassium concentrations should be determined before treatment because supplemental potassium in the presence of a high preexisting plasma level of potassium will intensify atrioventricular block and depress the automaticity of ectopic pacemakers in the ventricles, leading to complete heart block.
If renal function is normal and atrioventricular conduction block is not present, it is acceptable to administer potassium, 0.025 to 0.050 mEq/kg IV, to treat life-threatening cardiac dysrhythmias associated with digitalis toxicity, obviously with continuous ECG monitoring.
Phenytoin (0.5 to 1.5 mg/kg IV over 5 minutes) or lidocaine (1 to 2 mg/kg IV) is effective in suppressing ventricular cardiac dysrhythmias caused by digitalis.
Atropine, 35 to 70 μg/kg IV, can be administered to increase heart rate by offsetting excessive parasympathetic nervous system activity produced by toxic plasma concentrations of digitalis.
Propranolol is effective in suppressing increased automaticity produced by digitalis toxicity, but its tendency to increase atrioventricular node refractoriness limits its usefulness when conduction blockade is present.
Life-threatening digitalis toxicity can be treated by administering digoxin antibodies thus decreasing the plasma concentration of digoxin.
Drug Interactions
Quinidine produces a dose-dependent increase in the plasma concentration of digoxin that becomes apparent within 24 hours after the first dose. This effect of quinidine may be due to displacement of digoxin from binding sites in tissues.
Clinical experience does not support the occurrence of an increased incidence of cardiac dysrhythmias in patients being treated with cardiac glycosides and receiving succinylcholine.
Sympathomimetics with
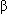 -adrenergic agonist effects may increase the likelihood of cardiac dysrhythmias in the presence of cardiac glycosides.
-adrenergic agonist effects may increase the likelihood of cardiac dysrhythmias in the presence of cardiac glycosides.IV administration of calcium may precipitate cardiac dysrhythmias in patients receiving cardiac glycosides.