- Verify the practitioner’s order, including the patient’s identifiers, the prescribed route based on the enteral tube’s tip location, enteral feeding device, prescribed enteral formula, administration method, volume and rate of administration, and type, volume, and frequency of water flushes.4
- Review the patient’s medical record to make sure that catheter placement was confirmed before beginning the feeding.4
- Gather and prepare the necessary equipment and supplies.
- Visually inspect the enteral formula for damage to the container, altered formula characteristics, and expiration date. Don’t use the formula if its integrity is compromised or if it’s expired; instead, obtain a new container of the formula.4
- Compare the label on the enteral formula container to the order in the patient’s medical record.4
- Perform hand hygiene.8,9,10,11,12,13
- Confirm the patient’s identity using at least two patient identifiers.14
- Provide privacy.15,16,17,18
- Explain the procedure to the patient and family (if appropriate) according to their individual communication and learning needs to increase their understanding, allay their fears, and enhance cooperation.19
- Raise the patient’s bed to waist level before providing care to prevent caregiver back strain.20
- Assess the patient to determine the risk for aspiration.4,21
- Position the patient with the head of the bed elevated to at least 30 degrees or upright in a chair to prevent aspiration. If this position is contraindicated, consider a reverse Trendelenburg position.4,22
- Perform hand hygiene.8,9,10,11,12,13
- Put on gloves to comply with standard precautions.23,24,25
For an Intermittent or a Continuous Feeding
- Open an enteral feeding bag with enteral administration set tubing if using an open administration system. If using a closed system, open the enteral administration set tubing.
- Pour only a premeasured 4-hour volume of enteral formula into the enteral feeding bag and hang the bag on the IV pole.4 Set infusion time for open enteral nutrition feeding systems to 4 to 8 hours.4 Limiting the volume decreases the risk of bacterial overgrowth in the formula. Alternatively, open the sterile enteral formula container and attach it to the enteral administration set.
- If using an enteral feeding pump, attach the enteral administration set tubing to the enteral feeding pump following the manufacturer’s instructions.
- Prime the enteral administration set according to the manufacturer’s recommendations to minimize air delivery into the GI tract.
- Make sure that the enteral formula container is labeled with the patient’s identifiers; formula name (and strength if diluted); date and time of formula preparation; date and time that the formula was hung; administration route, rate, and duration (if cycled or intermittent); initials of who prepared, hung, and checked the enteral formula against the order; expiration date and time; dosing weight (if appropriate); and notation ENTERAL USE ONLY-NOT FOR IV USE.4
- Label the enteral administration set with the date and time that it was first hung. Change the open-system administration set according to the manufacturer’s instructions to prevent bacterial growth. If you’re using a closed system, change the administration set according to the manufacturer’s instructions.4
- Place a fluid-impermeable pad or towel under the patient’s feeding tube to prevent soiling of linens.
- Verify tube placement by using at least two of the following methods.4,22,26 Observe for a change in the external length or incremental marking on the tube at the exit site to determine whether the tube has migrated.4,22,26 Observe for a change in volume of aspirate from the feeding tube, because a large increase in volume may signal the upward dislocation of a small-bowel feeding tube into the stomach; persistent inability to withdrawal fluid (or only a few drops of fluid) from the tube may signal upward displacement of a gastric tube into the esophagus.22,26 Review routine chest and abdominal X-ray reports.4,22,26 Aspirate tube contents and inspect the visual characteristics of the tube aspirate, because fasting gastric secretions often appear grassy-green or brown or clear and colorless.4,26 If performed at your facility, measure the pH of aspirate from the tube; fasting gastric pH is usually 5 or less, even in patients receiving gastric acid inhibitors.4,22,26
- If you suspect tube migration, don’t administer the enteral feeding. Instead, notify the practitioner.
- Flush the enteral feeding tube with at least 30 mL of water, as ordered. Use purified water for immunocompromised or critically ill patients.4
- Connect the end of the enteral administration set tubing to the distal end of the enteral tube. Trace the tubing from the patient to its point of origin to ensure that it’s connected to the proper port before beginning the tube feeding.4,27,28
- Tape the connection to prevent accidental disconnection of the tubing. Route the enteral administration set tubing toward the patient’s feet and place the enteral feeding pump toward the foot of the bed, because a standardized approach to keeping IV lines routed toward the head and enteric lines routed toward the feet prevents dangerous misconnections.27,28 If the patient has different access sites or several bags hanging, label each tubing at the distal end (near the patient connection) and proximal end (near the source container) to distinguish different tubing and prevent dangerous misconnections.28
- Open the administration set clamp. Regulate the flow to the desired rate. If using an enteral feeding pump, follow the manufacturer’s instructions for setting the flow rate and starting the infusion.4 Make sure that the enteral feeding pump alarm limits are set according to the patient’s current condition, and that alarms are turned on, functioning properly, and audible to staff.29,30,31
- Monitor the gravity drip rate or enteral feeding pump infusion rate frequently to ensure accurate delivery of the enteral formula.
- Flush the enteral feeding tube every 4 hours with at least 30 mL of water (purified water for immunocompromised or critically ill patients), as ordered and tolerated, to maintain patency and provide hydration.4
- Monitor the patient at least every 4 hours for appropriate positioning.4
- Assess for GI intolerance of enteral tube feedings every 4 hours by assessing for abdominal distention, monitoring for reports of abdominal pain, and observing for passage of flatus and stool.4,22 Gastric residual volume (GRV) may not need to be used routinely to monitor critically ill patients receiving enteral nutrition.4,21 For patient care areas that still monitor GRV, avoid holding the enteral feeding for a GRV of less than 500 mL if the patient has no other signs of feeding intolerance to prevent inappropriate stoppage of enteral feedings.4,32
- Perform oral care routinely to decrease oral bacterial colonization and, subsequently, reduce the risk of health care–acquired pneumonia.33 (See the "Oral care" procedure.)
- Remove and discard the fluid-impermeable pad or towel.25
- Monitor the patient’s weight and nutritional, fluid, electrolyte, and metabolic statuses, as ordered, to evaluate the effectiveness of enteral feedings.
For Site Care
- Perform hand hygiene.8,9,10,11,12,13
- Put on gloves to comply with standard precautions.23,24,25
- Gently remove the dressing to prevent skin stripping or tearing and discard it in an appropriate receptacle.19,34 Don’t cut away the dressing over the catheter, because you might cut the tube or the sutures holding the tube in place.
- Remove and discard your gloves.23,24
- Perform hand hygiene.8,9,10,11,12,13
- Put on a new pair of gloves.23,24
Gastrostomy or Jejunostomy Tube Site
- Assess the tube exit site for new or increasing pain and signs of skin breakdown, redness, edema, induration, and bleeding.4
- Inspect the tube for wear and tear. A tube that has worn out needs to be replaced.3,4
- Observe for a change in external tube length or the incremental marking at the exit site to assess for tube migration.3,4
- Until healing occurs, clean the skin immediately around the gastrostomy or jejunostomy tube’s exit site daily (and as needed) using a cotton-tipped applicator moistened with normal saline solution. Next, using a 4" × 4" (10 cm × 10 cm) gauze pad soaked in normal saline solution, clean the adjacent skin and pat it dry using another gauze pad. When the exit site has healed, wash the skin around it with soap and water daily. Rinse the area with water and pat it dry.3
- Apply skin protectant, if necessary, to prevent skin maceration.3
- Secure the gastrostomy or jejunostomy tube to the skin with and external stabilization device or hypoallergenic tape to prevent peristaltic migration of the tube and tension on the suture that anchors the tube in place.35
- Coil the tube, if necessary, and tape it to the abdomen to prevent pulling and contamination of the tube. Rotate the taping site to prevent skin damage.
PEG or PEJ Site Care
- Slide the tube’s outer bumper carefully away from the skin about ½" (1.3 cm). Depress the skin surrounding the tube gently and inspect for leakage. Minimal wound drainage, which appears initially after implantation, should subside in about 1 week.
- Assess the skin at the exit site for increasing pain and signs of infection, such as redness, edema, and purulent drainage.3
- Inspect the tube for wear and tear. A tube that is worn out needs to be replaced.3,4
- Observe for a change in external tube length or the incremental marking on the tube at the exit site to assess for tube migration.3,4
- Clean the exit site with soap and water-moistened gauze pads (as shown below), and allow it to dry.3,36,37
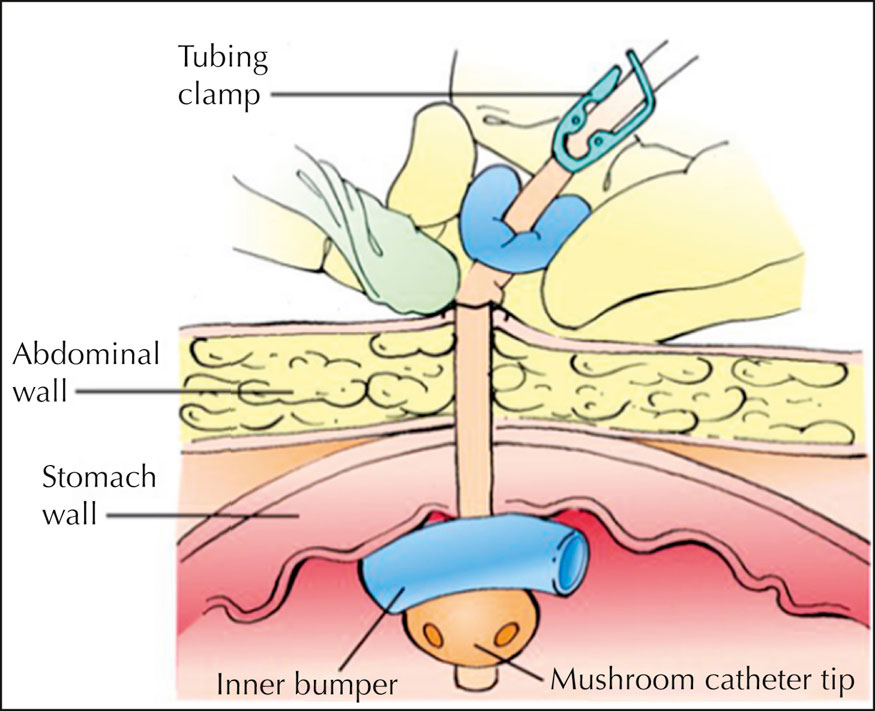
- Rotate the outer bumper 90 degrees to avoid applying the same tension to the same skin area and to prevent pressure injury formation at the exit site,38 and slide the bumper back over the exit site. Ensure that the outer bumper isn’t resting too tightly against the skin; one finger’s breadth should fit between the skin and the outer bumper.3
- If leakage appears at the PEG tube exit site, or if the patient risks dislodging the tube, apply a sterile gauze or foam dressing and an external stabilization device around the site, as needed.35 Apply the dressing over the outer bumper, because applying it underneath the outer bumper creates pressure on the gastrostomy tube tract, which could lead to wound abscess.37,39
- Label the dressing with the date, the time, and your initials.