
Definition
- Spontaneous hyperadrenocorticism (HAC) is a disorder caused by excessive production of cortisol by the adrenal cortex.
- Iatrogenic HAC results from excessive exogenous administration of glucocorticoids of any form or by any route.
- In either instance, clinical signs are due to deleterious effects of elevated circulating glucocorticoid concentrations on multiple organ systems.
Pathophysiology
- Approximately 80–85% of cases of naturally occurring HAC are due to bilateral adrenocortical hyperplasia resulting from pituitary corticotroph tumors or hyperplasia with oversecretion of ACTH.
- In the remaining 15–20% of cases, cortisol-secreting adrenocortical neoplasia is present; approximately one-half of these are malignant.
- Rarely caused by ectopic ACTH secretion from a non-pituitary tumor.
- Iatrogenic HAC results from excessive administration of exogenous glucocorticoids.
Systems Affected
- The degree to which each system is involved varies considerably; signs referable to one system may predominate or several systems may be involved to a comparable degree.
- Signs referable to the urinary tract or skin often predominate.
- Endocrine/Metabolic-hyperglycemia; diabetes mellitus occurs in 10%.
- Cardiovascular-hypertension (usually mild).
- Gastrointestinal-polyphagia.
- Hemic/Lymphatic/Immune-stress leukogram; immunosuppression; mild erythrocytosis and thrombocytosis.
- Hepatobiliary-hepatopathy due to glycogen deposition; increased serum ALP activity due to production of corticosteroid-induced isoenzyme.
- Neuromuscular-muscle weakness; CNS signs including anorexia, ataxia, disorientation and, uncommonly, seizures if pituitary macroadenoma present.
- Renal/Urologic-polyuria/polydipsia in 90% of cases; proteinuria; UTI common.
- Reproductive-testicular atrophy and anestrus.
- Respiratory-panting; pulmonary thromboembolism possible due to a hypercoagulable state.
- Skin-bilaterally symmetric alopecia common; comedones; hyperpigmentation; recurrent pyoderma.
Genetics
No genetic basis known.
Incidence/Prevalence
- No exact figures available.
- Considered one of most common endocrine disorders in dogs.
Signalment
Species
Dog
Breed Predilections
Poodle, dachshund, Boston terrier, German shepherd dog, and beagle.
Mean Age and Range
Generally a disorder of middle-aged to old animals; pituitary-dependent HAC (PDH) can very rarely be seen in dogs as young as 1 year.
Predominant Sex
No predilection for PDH in dogs; possible predilection for female dogs to have an adrenal tumor.
Signs
General Comments
- Severity varies greatly, depending on duration and severity of cortisol excess.
- In some cases, the physical presence of the neoplastic process (pituitary or adrenal) contributes.
Historical and Physical Examination Findings
Polyuria and polydipsia, polyphagia, pendulous abdomen, increased panting, hepatomegaly, hair loss, cutaneous hyperpigmentation, thin skin, muscle weakness, obesity, lethargy, muscle atrophy, comedones, bruising, testicular atrophy, anestrus, calcinosis cutis, facial nerve palsy.
Causes
- Pituitary-dependent-adenoma most common; adenocarcinomas rare; anterior pituitary involved in approximately 80% of cases, intermediate lobe in remaining cases; exact incidence of pituitary macroadenomas (i.e., >1 cm diameter) unknown, may be 10–25%.
- Adrenal tumor-adenoma or carcinoma (50/50).
- Ectopic ACTH secretion-rare.
- Iatrogenic-due to glucocorticoid administration.
Risk Factors
- None known for spontaneous disease.
- Presence any condition that leads to exogenous glucocorticoid administration is a risk factor for iatrogenic HAC.

 10% chance).
10% chance).

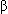 -hydroxysteroid dehydrogenase and maybe others, thereby suppressing production of
-hydroxysteroid dehydrogenase and maybe others, thereby suppressing production of 
