
Overview
- Acquired or hereditary defects that can affect any of the main functions of platelets, including procoagulant activity. Defects are divided in 2 categories: intrinsic and extrinsic (e.g., vWD).
- Affected animals typically have normal platelet counts but have spontaneous or excessive bleeding; mucosal bleeding is the most common sign.
- Thrombocytopenic animals with concurrent thrombocytopathia will bleed more excessively than expected for the platelet count.
Signalment
- Acquired defects are the most common thrombocytopathias seen in companion animals. They occur in all breeds and at all ages.
- Hereditary platelet function defects may be diagnosed at all ages but may first appear in young animals when excessive bleeding occurs with loss of deciduous teeth.
- Hereditary defects are rare disorders that have been described in the following breeds/species:
- Type I Glanzmann thrombasthenia-otter hound and Great Pyrenees
- Storage pool disease-Chediak-Higashi syndrome-Persian cat; delta-granule-American cocker spaniel; cyclic hematopoiesis-grey collie
- Thrombocytopathia (CalDAG-GEFI deficiency) -basset hound, spitz, and Landseer
- Scott syndrome-German shepherd
- P2Y12 (ADP) receptor mutation-Greater Swiss mountain dog
Signs
- Often mild spontaneous muco-cutaneous bleeding, such as epistaxis, petecchiation, and gingival bleeding.
- Prolonged bleeding in some animals during or following diagnostic or surgical procedures.
- In Scott syndrome, postoperative hemorrhage is the most common sign.
Causes & Risk Factors
Acquired-Drugs
- NSAIDs (e.g., aspirin) inhibit platelet function by preventing the formation of thromboxane A2. This effect is less pronounced to absent with more selective cyclooxygenase-2 antagonists (e.g., meloxicam, deracoxib).
- Potentiated sulfonamides and hydroxyethyl starch solutions suppress platelet function in dogs.
- Penicillins, tetracyclines, anesthetic/sedative agents, and antihistamines cause thrombocytopenia and/or platelet function defects in humans-however, effects have not been documented in dogs and cats.
Secondary to Systemic Disease
Dissemininated intravascular coagulopathy, uremia, anemia, liver disease (cholestasis and acquired or inherited shunts), ehrlichiosis, leishmaniasis, immune-mediated thrombocytopenia, heart disease, and neoplastic disorders (both hematopoietic and non-hematopoietic neoplasms).
Hereditary
- von Willebrand disease-a deficiency (type I and III) or qualitative defect (type II) of von Willebrand factor.
- Basset hound, spitz, and Landseer hereditary thrombocytopathia-signal transduction defects due to CalDAG-GEFI mutations.
- Otter hound and Great Pyrenees with type I Glanzmann thrombasthenia-platelet defect caused by a mutation in glycoprotein IIb-IIIa (integrin
 Iib
Iib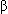 3) receptor on the platelet surface.
3) receptor on the platelet surface. - Storage pool disease: Chediak-Higashi syndrome and deficiency in delta-granule storage pool of ADP-aggregation defect caused by lack of adenine nucleotides.
- Scott syndrome in German shepherd-platelet procoagulant deficiency (failure to externalize phospatidyl-serine on the platelet surface and inability of the platelets to support effective assembly of coagulation complexes).
- P2Y12 (ADP) receptor mutation in Greater Swiss mountain dog.




