
Overview
Ventricular tachycardia may occur in structurally normal hearts (hereditary arrhythmias) or may occur due to myocardial abnormalities associated with cardiomyopathy, significant valvular disease, or myocarditis. To date, there is no medical therapy available that is known to prevent sudden death in animals afflicted with ventricular tachyarrhythmias.
ECG Features
- Three or more ventricular premature contractions in a row.
- May be intermittent (paroxysmal) or sustained; heart rate usually >150 bpm with a regular or irregular rhythm.
- QRS complexes-typically wide and bizarre.
- If P waves visible-dissociated from the QRS complexes.
- Breed-specific ECG changes-VT in boxers is characteristically positive in the ventrocaudal leads (leads II, III, and aVF) or display a “left bundle branch block pattern.” VT in Doberman pinschers and German shepherds has both polymorphic and monomorphic characteristics.
Pathophysiology
Potentially life-threatening arrhythmia because it can degenerate into ventricular fibrillation, resulting in sudden death. Usually signifies underlying myocardial disease or metabolic/electrolyte derangement; mechanisms include increased automaticity, reentry, and triggered activity.
Systems Affected
Cardiovascular system, with secondary effects on other systems because of poor perfusion.
Genetics
- Arrhythmogenic right ventricular cardiomyopathy (ARVC) in boxer dogs and dilated cardiomyopathy with VT in Doberman pinschers are both inherited as autosomal dominant traits.
- There is an association with a striatin mutation with the development of DCM in boxers. Striatin is desmosomal protein (scaffolding protein) that has been associated with ARVC in humans. In addition, the Wnt signaling pathway is now implicated in boxer dogs with ARVC. In brief, WnT signaling pathways play essential roles in in cell behavior, survival, and proliferation.
- There is a mutation in Doberman pinschers in the PDK4 gene. However, there are more than 20 different mutations in humans that can cause DCM.
- Ventricular arrhythmias and sudden cardiac death are hereditary in German shepherd dogs; mode of inheritance is polygenic due to an abnormality in a major gene with modifiers.
- In the Maine Coon and Ragdoll cat a mutation in the myosin binding protein C (MYBPC3) has been identified. However, the Ragdoll mutation is different from the Maine Coon mutation as it is located in a different region of the gene.
- Testing positive for the genetic defect does not mean all animals will express the phenotype of the disease.
Incidence/Prevalence
Common arrhythmia in dogs; uncommon in cats
Geographic Distribution
None
Signalment
Species
Dogs and cats
Breed Predilections
Commonly seen in large-breed dogs with cardiomyopathy, especially boxers and Doberman pinschers. German shepherd dogs with sudden cardiac death.
Mean Age and Range
- All age groups if not breed-specific VT.
- Boxers with arrhythmogenic cardiomyopathy usually present at 4–6 years of age, frequency and severity of the arrhythmia usually increases over time.
- Doberman pinschers with occult cardiomyopathy typically develop ventricular arrhythmias beginning at 3–6 years of age, but it also can occur much later in life; frequency and severity of the arrhythmia usually increases over time.
- German shepherds develop ventricular arrhythmias at 12–16 weeks of age and the frequency and severity of the arrhythmias increases until 24–30 weeks of age. After 8 months of age the arrhythmia severity stabilizes or starts to decrease.
Signs
Historical Findings
- Syncope
- Weakness
- Exercise intolerance
- Sudden death
- May be asymptomatic
Physical Examination Findings
- May be normal if arrhythmia is paroxysmal and absent during examination.
- Paroxysmal or sustained tachycardia may be ausculted.
- Femoral pulses may have variable pulse intensity or are weak during runs of VT.
- Signs of congestive heart failure or murmur may be present, depending on cause of arrhythmia.
Causes
- Cardiomyopathy
- Congenital defects (especially subaortic stenosis)
- Chronic degenerative valve disease
- Traumatic or infectious myocarditis
- Cardiac neoplasia
- Gastric dilation and volvulus
- Splenic neoplasia/hemorrhage
- Hyperthyroidism (cats)
- Digitalis toxicity
- Pancreatitis
Risk Factors
- Hypokalemia, hyperkalemia
- Hypomagnesemia
- Acid-base disturbances
- Hypoxemia
- Neoplasia (e.g., cardiac or splenic hemangiosarcoma)
- Anemia





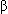 -catenin localization suggests involvement of the canonical Wnt pathway in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy
-catenin localization suggests involvement of the canonical Wnt pathway in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy