Definition
- Osmolarity-expressed in mOsm/L; represents the number of solute particles per liter of solution.
- Osmolality-expressed in mOsm/kg; represents the number of solute particles per kilogram of solution.
- Hyperosmolarity-a high concentration of solute particles per liter of solution.
- Serum concentrations >310 mOsm/L in dogs and >330 mOsm/L in cats are usually considered hyperosmolar.
- Morbidity from hyperosmolarity is related more to rapid changes in osmolarity than to the actual amount of change.
Pathophysiology
- Serum sodium is responsible for most of the osmotically active particles that contribute to serum osmolarity; serum glucose and urea also contribute to serum osmolarity.
- Anything that causes water loss increases concentrations of solutes in plasma or serum, thereby increasing serum osmolarity.
- Blood volume, hydration status, and ADH are intimately involved in controlling extracellular fluid volume.
- Low circulating blood volume stimulates carotid and aortic baroreceptors to respond to changes in blood pressure, causing ADH secretion.
- Hyperosmolarity affects the osmoreceptors in the hypothalamus and stimulates ADH secretion from the neurohypophysis; the hypothalamic thirst center is also stimulated and causes an increase in water consumption to counteract serum hyperosmolarity by solute dilution.
- Rapid increases in serum osmolarity cause water movement along its concentration gradient from intracellular to extracellular spaces, resulting in neuronal dehydration, cell shrinkage, and cell death; cerebral vessels may weaken and hemorrhage.
Systems Affected
- Cardiovascular-hypotension and decreased ventricular contractility.
- Nervous-excessive thirst may be the first sign of hyperosmolarity. Central nervous system depression may lead to coma.
- Renal/Urologic-low urine output.
Signalment
- Dog and cat.
- Hypodipsia and hyperosmolarity have been reported in young female miniature schnauzers.
Signs
General Comments
- Primarily neurologic or behavioral.
- Severity is related more to how quickly hyperosmolarity occurs than to the absolute magnitude of change.
- Most likely to occur if serum osmolarity is >350 mOsm/L and usually severe if >375 mOsm/L.
Historical Findings
Anorexia, lethargy, vomiting, weakness, disorientation, ataxia, seizures, and coma; polydipsia followed by hypodipsia.
Physical Examination Findings
- Normal, or abnormalities may reflect underlying disease.
- In addition to historical findings, dehydration, tachycardia, hypotension, weak pulses, and fever may be detected.
Causes
Increased Solutes
Hypernatremia, hyperglycemia, severe azotemia, ethylene glycol toxicosis, salt poisoning, sodium phosphate enemas in cats and small dogs, mannitol, radiographic contrast solution, administration of ethanol, aspirin toxicosis, shock, lactate in patients with lactic acidosis, acetoacetate and 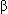 -hydroxybutyrate in patients with ketoacidosis, liquid enteral nutrition, and parenteral nutrition solutions.
-hydroxybutyrate in patients with ketoacidosis, liquid enteral nutrition, and parenteral nutrition solutions.
Decreased Extracellular Fluid Volume
Dehydration-gastrointestinal loss, cutaneous loss, third space loss, low water consumption, and polyuria without adequate compensatory polydipsia.
Risk Factors
- Medical conditions that predispose-renal failure, diabetes insipidus, diabetes mellitus, hyperadrenocorticism, hyperaldosteronism, and heat stroke.
- Therapeutic hyperosmolar solutions-hypertonic saline, sodium bicarbonate, sodium phosphate enemas in cats and small dogs, mannitol, and parenteral nutrition solutions.
- High environmental temperatures.
- Fever.




