
Definition
- Neutrophil count <3,000 neutrophils/µL in dogs and <2,500 neutrophils/µL in cats.
- Can develop alone or as a component of pancytopenia.
- Often accompanied by a left shift and toxic changes (e.g., cytoplasmic basophilia, foamy cytoplasmic vacuolation, Döhle bodies, and/or toxic granulation).
- Certain breeds, such as greyhounds or Belgian Tervurens, can normally have a neutrophil count below the reference interval for other dogs.
Pathophysiology
Results from one of four mechanisms-(1) decreased production or release of neutrophils from the bone marrow, (2) a shift of neutrophils from the circulating pool within the large vessels to the marginating pool, adhered to the endothelium of capillaries, (3) increased migration into the tissues from the blood due to severe inflammation/tissue consumption, and (4) immune-mediated destruction.
Systems Affected
- Predisposes the patient to systemic infection by a variety of pathogens.
- Many body systems can be affected in any combination, depending on the site(s) of infection.
Genetics
- Canine cyclic hematopoiesis is autosomal recessive, involving the adaptor protein complex 3 (AP3)
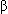 -subunit, which redirects neutrophil elastase trafficking from membranes to granules.
-subunit, which redirects neutrophil elastase trafficking from membranes to granules. - A genetic trait with delayed penetrance seems likely to explain the age-related neutropenia in Belgian Tervurens.
- Selective cobalamin malabsorption is autosomal recessive, resulting in neutropenia due to failure to express the receptor for intrinsic factor-cobalamin complex. A CUBN frameshift mutation is likely causative for Imerslund-Gräsbeck Syndrome (selective cobalamin malabsorption) in border collies.
- An autosomal recessive neutropenia, known as trapped neutrophil syndrome (TNS), has been seen in Australian and New Zealand border collies with a deficiency of segmented neutrophils in the blood and hyperplasia of myeloid cells in the bone marrow due to an alternately spliced transcript of VPS13B, similar to Cohen syndrome in humans.
Signalment
- Nothing specific for generalized infection.
- Schnauzers, beagles, Australian shepherds, Shar-Peis, and border collies with inherited cobalamin malabsorption.
- Gray collies and possibly border collies with canine cyclic hematopoiesis.
- Belgian Tervuren dogs.
- Related border collies in Australia and New Zealand with chronic neutropenia.
- G-CSF deficiency has been reported in a rottweiler with chronic idiopathic neutropenia.
Signs
- Septic animals usually present with nonspecific signs of illness such as lethargy, weakness, and inappetence. They are often febrile, but normothermia does not rule out infection. Other signs can include tachycardia, injected mucous membranes, prolonged capillary refill time, and weak pulses, some of which are related to septicemia and endotoxic shock.
- Gray collies with cyclic hematopoiesis exhibit severe neutropenia every 12–14 days. Episodes of fever, diarrhea, gingivitis, respiratory infection, lymphadenitis, and arthritis occur in association with the neutropenia. These dogs seldom live past 1 year of age.
- No clinical signs in Belgian Tervurens.
- Related border collies in Australia and New Zealand with chronic neutropenia had recurrent bacterial infections manifesting as osteomyelitis and gastroenteritis. A dog with heat stroke demonstrated inappropriate rubricytosis with a high normal PCV, moderate mature neutropenia and lymphopenia, and petechiation despite only mild thrombocytopenia. Numerous leukocytes showed evidence of apoptosis; many neutrophils had botryoid nuclei.
Causes
Deficient Neutrophil Production, Stem Cell Death, or Inhibition
- Infectious agents-dogs and cats, parvoviruses, bacteria-induced myelonecrosis, and systemic mycosis; cats, FeLV and FIV; dogs, monocytic and granulocytic ehrlichiosis, Babesial infections.
- Drugs, chemicals, and toxins-dogs and cats, chemotherapy agents and cephalosporins; cats, T-2 mycotoxin ingestion, chloramphenicol and benzene-ring compounds, methimazole, and griseofulvin; dogs, estrogen, phenylbutazone, trimethoprim-sulfadiazine, phenobarbital.
- Lack of trophic factors-inherited malabsorption of cobalamin/vitamin B12.
- Ionizing radiation.
Reduced Hematopoietic Space Secondary to Myelophthisis
- Myelonecrosis
- Myelofibrosis
- Disseminated neoplasia, leukemia, and myelodysplastic syndrome
- Disseminated granulomatous disease (histoplasmosis and cryptococcosis)
Cyclic Stem Cell Proliferation
- Inherited cyclic hematopoiesis
- Cyclophosphamide treatment
- Idiopathic disease
- Immune-mediated suppression of granulopoiesis
- Poorly documented in dogs and cats
Neutrophil Migration
A shift in neutrophils from the circulating neutrophil pool to the marginating neutrophil pool occurs in patients with endotoxemia. Thought to be the mechanism behind neutropenia in septicemic animals due to Bartonella spp. and other bacteria. During the acute phase, neutropenia suggests intense tissue recruitment in response to endothelial damage caused by Rangelia vitalii infection. Anaphylaxis is another, although uncommon, cause.
Reduced Survival
- Severe bacterial infection (most common cause)-sepsis, pneumonia, peritonitis, and pyothorax.
- Immune-mediated destruction (uncommon).
- Drug-induced destruction.
- Hypersplenism (sequestration).
- Neutropenia likely due to oncotic and apoptotic death of mature neutrophils in heat stroke.
Risk Factors
- Inherited disease-cyclic hematopoiesis in Gray collies and possibly border collies.
- Inherited cobalamin malabsorption in giant schnauzers, beagles, Australian shepherds, and border collies.
- Drug and chemical exposure-estrogen overdose in dogs (pancytopenia) and chloramphenicol and benzene-ring compounds in cats.
- Exposure to various infectious agents-dogs and cats, overwhelming bacterial infection; dogs, acute infection with Ehrlichia canis, parvovirus, Babesia canis rossi (travel history to/from South Africa), Rangelia vitalii (travel history to/from Brazil), and Anaplasma phagocytophilum (more likely lymphopenic and eosinopenic than neutropenic); cats, panleukopenia, FIV, and FeLV infection.
- Middle-aged and old animals are less effective at repopulating the bone marrow after a severe toxic insult.




