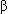 (1, 3)-D-glucan, a necessary component of the fungal cell wall.
(1, 3)-D-glucan, a necessary component of the fungal cell wall.Absorption: IV administration results in complete bioavailability.
Distribution: Widely distributed to tissues.
Protein Binding: 97%.
Metabolism/Excretion: Slowly and extensively metabolized; <1.5% excreted unchanged in urine.
Half-life: Polyphasic: 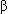 phase: 9–11 hr;
phase: 9–11 hr; 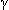 phase: 40–50 hr.
phase: 40–50 hr.
Contraindicated in:
Use Cautiously in:
Derm: flushing, pruritus, rash, STEVENS-JOHNSON SYNDROME (SJS), TOXIC EPIDERMAL NECROLYSIS.
GI: ↑ liver enzymes, diarrhea, nausea, vomiting.
GU: ↑ serum creatinine.
Local: venous irritation at injection site.
Neuro: headache.
Resp: bronchospasm.
Misc: chills, fever, HYPERSENSITIVITY REACTIONS (INCLUDING ANAPHYLAXIS AND ANGIOEDEMA).
Drug-Drug:
Hepatic Impairment
IV Administration:
NDC Code*