Drug-Drug:
- ↑ likelihood of hypoglycemia with short-acting insulin; ↓ dose of short-acting pre-meal insulin by 50%.
- Avoid concurrent use with other agents that ↓ GI motility, including atropine and other anticholinergics.
- Avoid concurrent use with other agents that ↓ GI absorption of nutrients, including
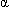 -glucosidase inhibitors including acarbose and miglitol.
-glucosidase inhibitors including acarbose and miglitol. - May delay oral absorption of concurrently administered drugs; if prompt absorption is desired, administer 1 hr before or 2 hr after pramlintide.
