- PO (Adults and Children
 13 yr): 300 mg every 12 hr or 600 mg every 24 hr (twice daily dosing must be used for community-acquired pneumonia or uncomplicated skin and skin structure infections).
13 yr): 300 mg every 12 hr or 600 mg every 24 hr (twice daily dosing must be used for community-acquired pneumonia or uncomplicated skin and skin structure infections).
Renal Impairment
- (Adults and Children
 13 yr): CCr <30 mL/min — 300 mg every 24 hr.
13 yr): CCr <30 mL/min — 300 mg every 24 hr. - PO (Children 6 mo–12 yr): 7 mg/kg every 12 hr or 14 mg/kg every 24 hr (not to exceed 600 mg/day). Twice daily dosing must be used for uncomplicated skin and skin structure infections.
Renal Impairment
- (Children 6 mo-12 yr): CCr <30 mL/min — 7 mg/kg every 24 hr.

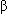 -lactamase-producing strains),
-lactamase-producing strains),