An accurate staging system is crucial in cancer management for predicting prognosis, guiding clinicians in treatment decisions for different risk groups, and sharing experience on results of treatment between centers. Prognostic significance of staging system changes with advances in investigation and treatment methods. Evaluation of staging systems to ensure continual suitability and exploration for further improvement is essential.
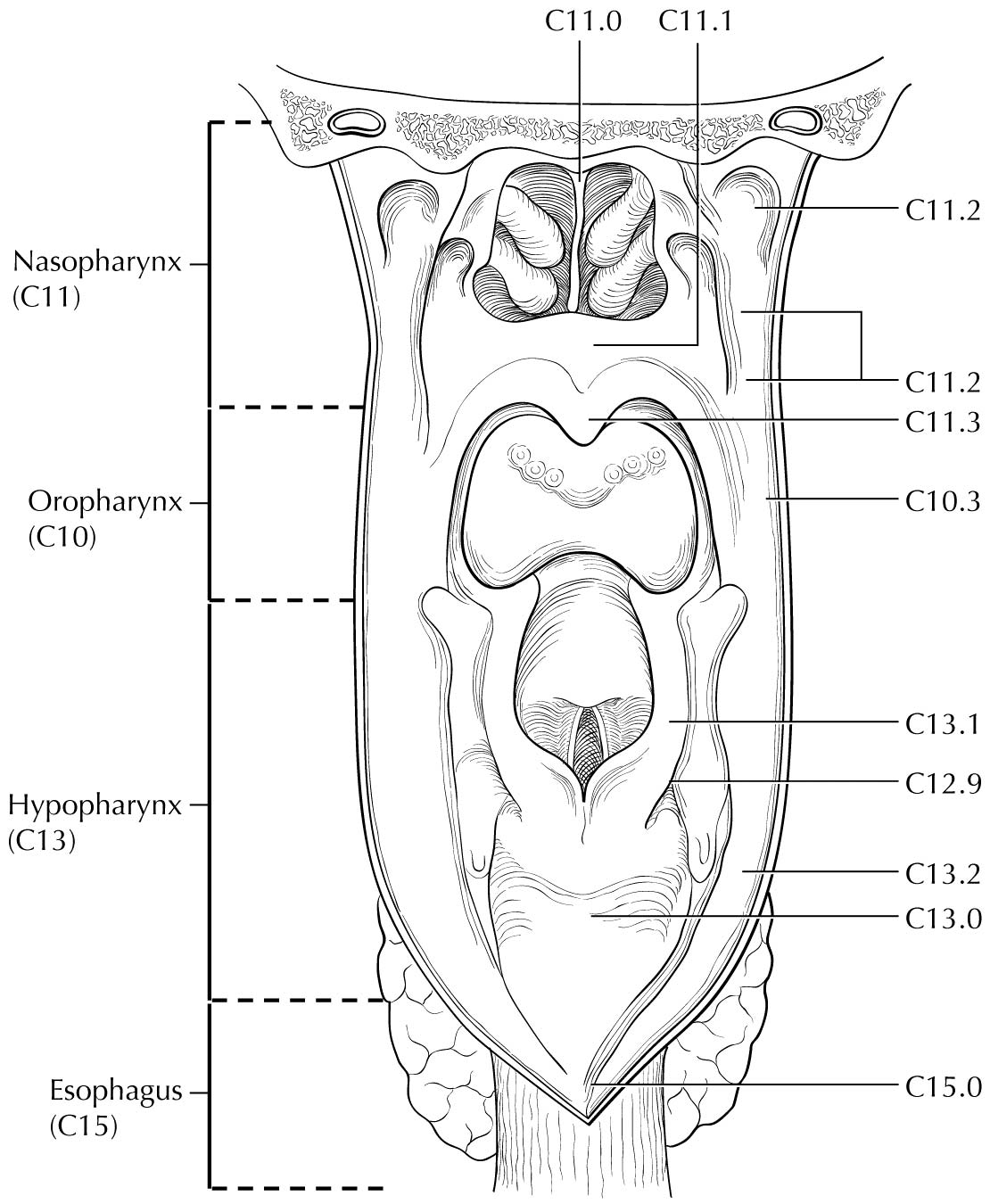
This chapter focuses on TNM staging for epithelial tumors of the nasopharynx. Nonepithelial tumors such as mucosal melanoma, lymphoma, and sarcoma of soft tissue, bone, and cartilage are not included.Nasopharyngeal carcinoma (NPC) has a very skewed geographic and ethnic distribution, with 80% of the global burden in Asian countries. The natural behavior and therapeutic consideration for NPC are different from other head and neck cancers. The adoption of a customized system for NPC in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 5th Edition, by the AJCC and the Union for International Cancer Control (UICC) was a milestone.1,2 The staging criteria were developed by merging the strengths of the AJCC/UICC, 4th Edition, and the Ho's System from Hong Kong.3,4 This development has gained global acceptance as studies from different countries (endemic and nonendemic) consistently showed substantial improvement as compared with prior systems. Almost all countries, except China, had adopted this international system.
No change was recommended in the AJCC Cancer Staging Manual, 6th Edition5,6 except for addition of the term “masticator space” as a synonym for “infratemporal fossa” (one of the T4 criteria) because although the intended extent was described in the staging hand book, the latter was not a clearly defined space with universal acceptance. Both terms were retained as T4 criteria in the AJCC Cancer Staging Manual, 7th Edition (7th Edition);7,8 however, the term “masticator space” was described using the boundaries stated in classical anatomy textbooks instead of the demarcation used for “infratemporal fossa.” Additional changes included down-shifting of tumors with extension to nasal fossa/oropharynx without parapharyngeal extension (previously T2a) to T19 and clear definition of retropharyngeal lymph node(s) involvement (unilateral or bilateral) as N1.10
The management of NPC has undergone substantial evolution in the past two decades. More accurate imaging methods have allowed better delineation of tumor extent and early detection of occult metastases. The advances in radiotherapy technique has led to increasing conformity of tumor coverage and sparing of noninvolved structures. The use of combination chemotherapy has further improved tumor control and cure rates, especially for advanced locoregional disease. It is therefore important that the new staging system be based on data from patients managed with contemporary methods.
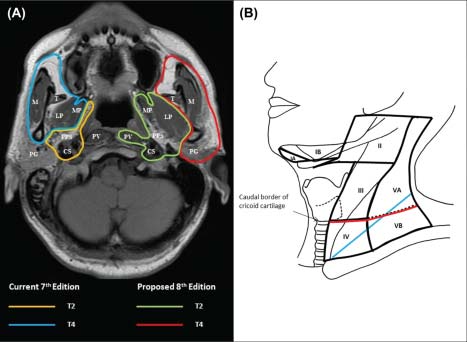
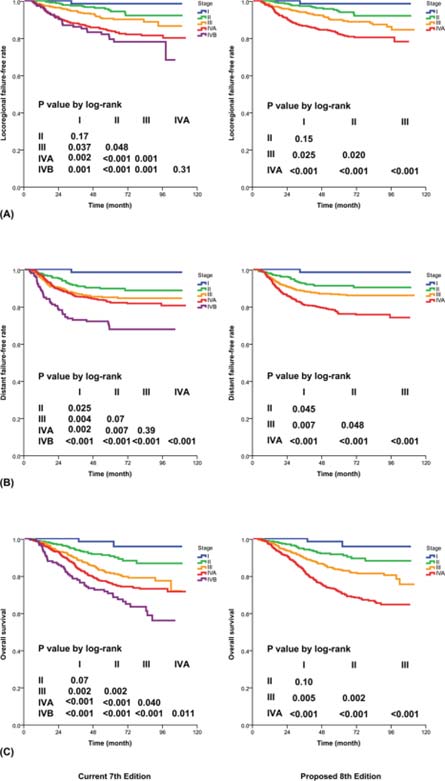
Extensive literature review showed that there are four major issues for consideration of improvement: (1) the controversy about the significance of “masticator space”,11-16 (2) uncertainty about the significance of prevertebral muscle invasion,17-19 (3) the possibility of replacing supraclavicular fossa (SCF)3 with anatomic nodal “levels,”20-25 and (4) simplification by elimination of unnecessary subgroups.25,26 These suggestions were validated by a large series of patients who were staged with magnetic resonance (MR) imaging and treated with intensity-modulated radiotherapy ± chemotherapy from two major centers (in Hong Kong and Fujian, China),27 before attaining consensus among international multidisciplinary experts. The strengths of the 7th Edition and the Chinese 2008 staging system23,24 are incorporated in developing the staging criteria in this AJCC Cancer Staging Manual, 8th Edition (8th Edition).