Primary Site(s)
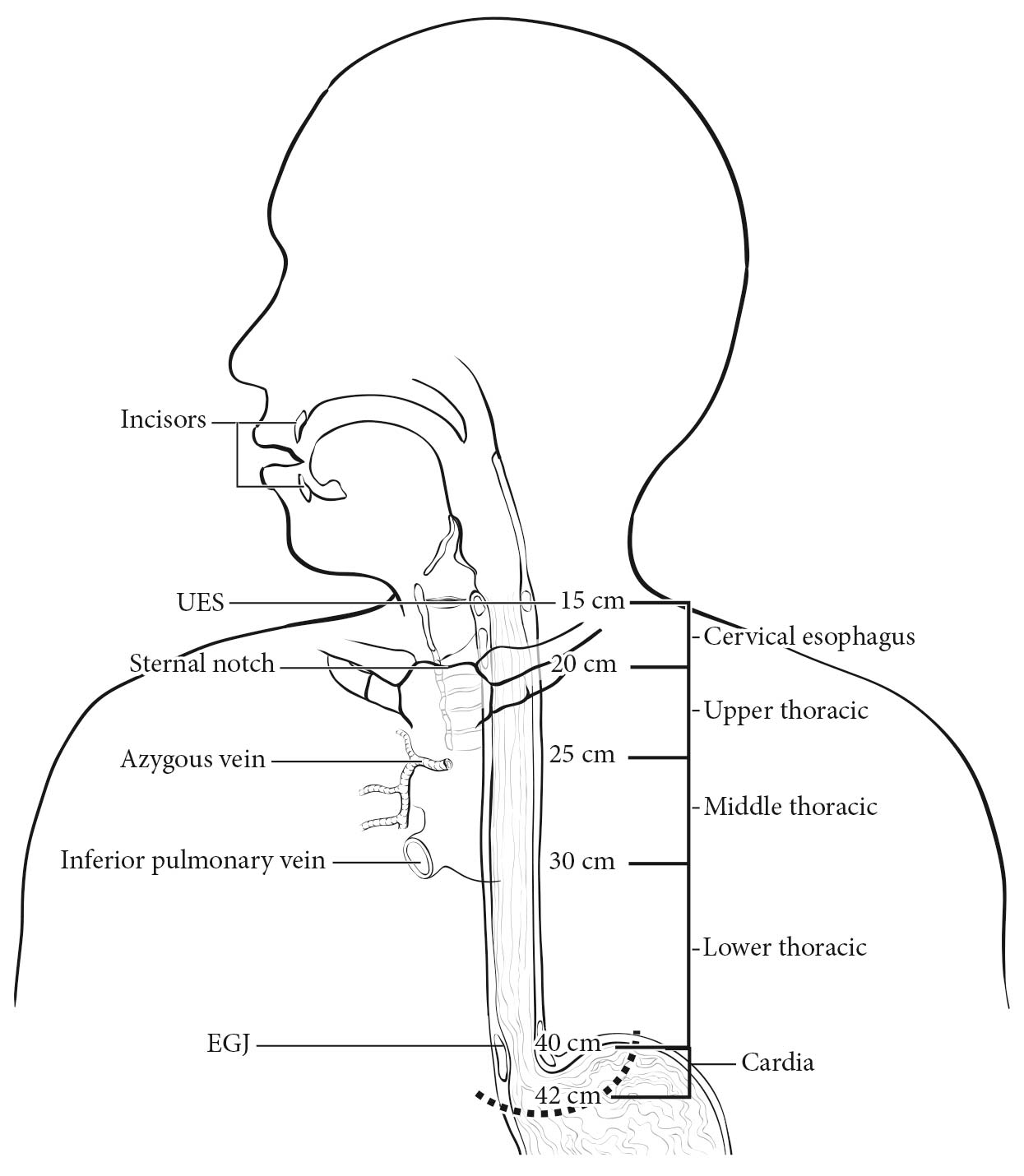
The esophagus traverses three anatomic compartments: cervical, thoracic, and abdominal. The thoracic esophagus is divided arbitrarily into equal thirds: upper, middle, and lower (Table 22.1). However, the clinical importance of the primary site of an esophageal cancer is related less to its position in the esophagus than to its relation to adjacent structures (Figure 22.1).
22.1 Anatomy of esophageal cancer primary site, including typical endoscopic measurements of each region measured from the incisors. Exact measurements depend on body size and height. Location of cancer primary site is defined by cancer epicenter. EGJ, esophagogastric junction; LES, lower esophageal sphincter; UES, upper esophageal sphincter.
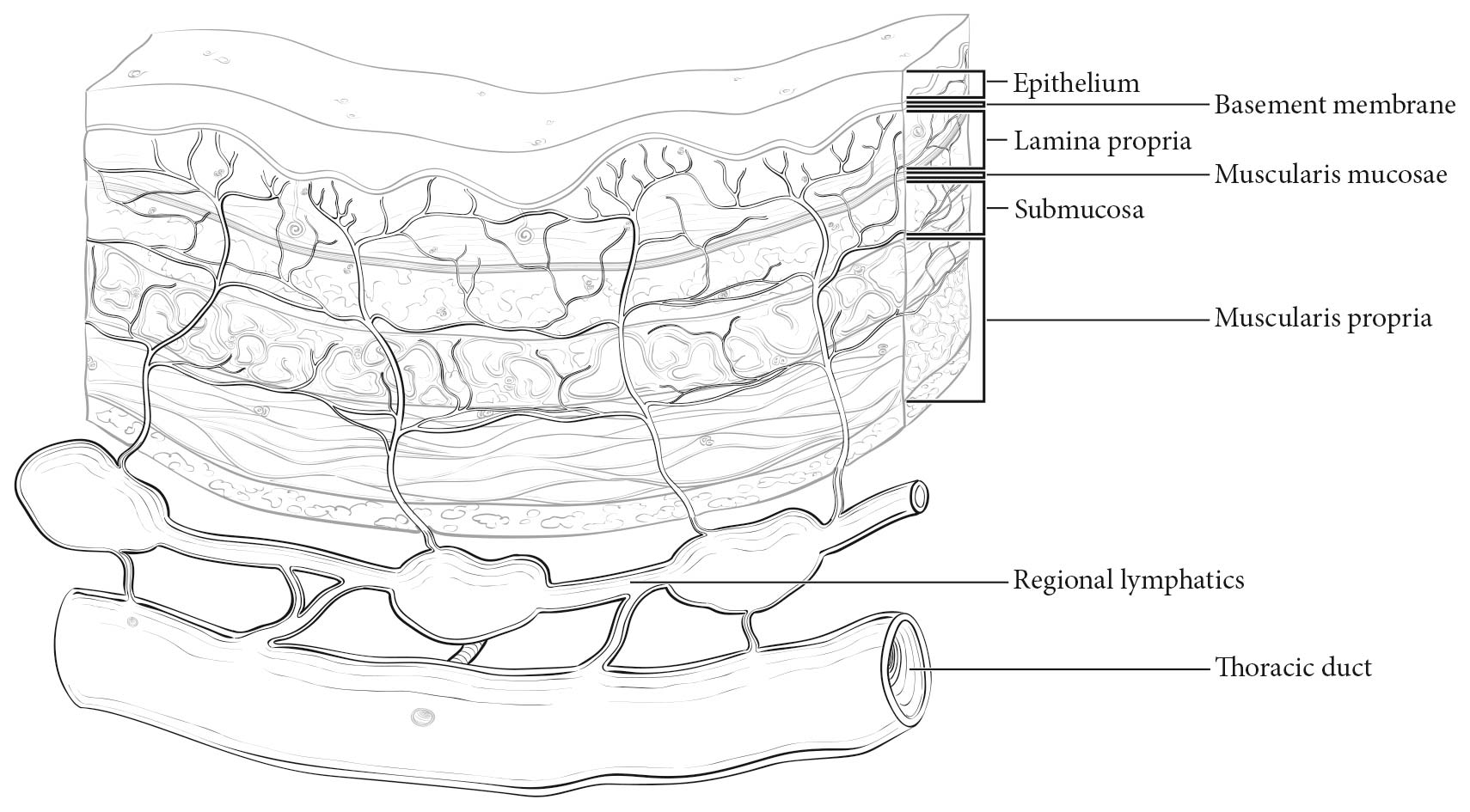
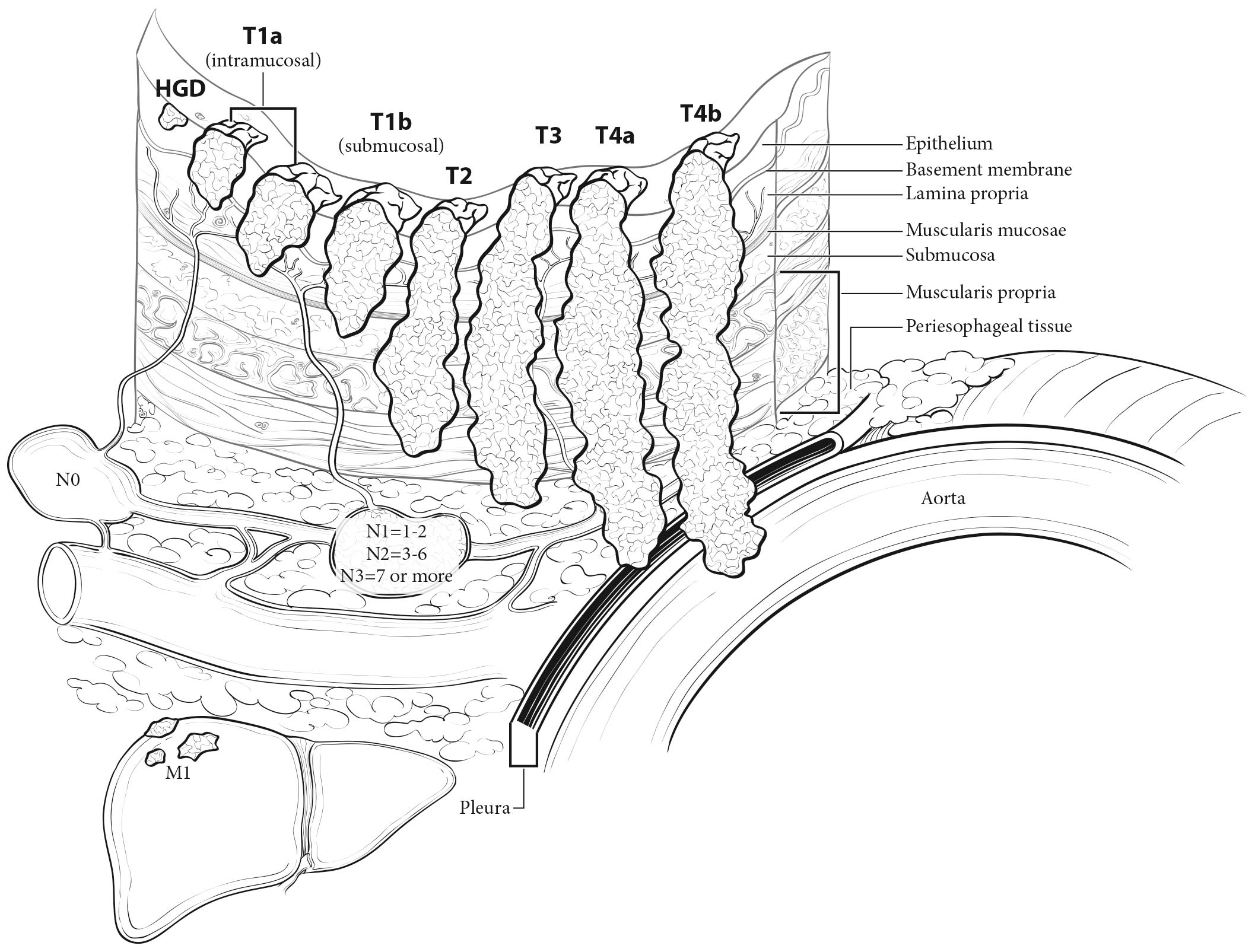
The esophageal wall has three layers: mucosa, submucosa, and muscularis propria (Figure 22.2). The mucosa is composed of epithelium, lamina propria, and muscularis mucosae. A basement membrane isolates the epithelium from the rest of the esophageal wall. In the columnar-lined esophagus, the muscularis mucosae may be a two-layered (duplicated) structure. The clinical importance of this duplicate layer is questionable.7,8 The outer layer is considered the true boundary. The mucosal division may be classified as m1 (epithelium), m2 (lamina propria), or m3 (muscularis mucosae).9 The submucosa has no landmarks, but it may be divided into inner (sm1), middle (sm2), and outer (sm3) thirds.9 The muscularis propria has inner circular and outer longitudinal muscle layers. There is no serosa; rather, adventitia (periesophageal connective tissue) lies directly on the muscularis propria.
Location
Cervical Esophagus
Cancers located in the cervical esophagus are staged as upper thoracic esophageal cancers, not as head and neck cancers.
Anatomically, the cervical esophagus lies in the neck, bordered superiorly by the hypopharynx and inferiorly by the thoracic inlet, which lies at the level of the sternal notch. It is subtended by the trachea, carotid sheaths, and vertebrae. Although the length of the esophagus differs somewhat with body habitus, gender, and age, typical endoscopic measurements for the cervical esophagus measured from the incisors are from 15 to less than 20 cm (Figure 22.1). If esophagoscopy is not available, location may be assessed by computed tomography (CT). If the epicenter of the tumor begins above the sternal notch, the location is defined as cervical esophagus.
Upper Thoracic Esophagus
The upper thoracic esophagus is bordered superiorly by the thoracic inlet and inferiorly by the lower border of the azygos vein. Anterolaterally, it is surrounded by the trachea, aortic arch, and great vessels, posteriorly by the vertebrae. Typical endoscopic measurements from the incisor teeth are from 20 to less than 25 cm (Figure 22.1). On CT, to determine the location, the epicenter of an upper thoracic cancer is visible between the sternal notch and the azygos vein.
Middle Thoracic Esophagus
The middle thoracic esophagus is bordered superiorly by the lower border of the azygos vein and inferiorly by the lower border of the inferior pulmonary vein. It is sandwiched between the pulmonary hilum anteriorly, descending thoracic aorta on the left, and vertebrae posteriorly; on the right, it lies freely on the pleura. Typical endoscopic measurements from the incisors are from 25 to less than 30 cm (Figure 22.1). On CT, to determine the location, the epicenter of a middle thoracic cancer is between the azygos vein and the inferior pulmonary vein.
Lower Thoracic Esophagus/Esophagogastic Junction (EGJ)
The lower thoracic esophagus is bordered superiorly by the lower border of the inferior pulmonary vein and inferiorly by the stomach. It is bordered anteriorly by the pericardium, posteriorly by vertebrae, and on the left by the descending thoracic aorta. It normally passes through the diaphragm to reach the stomach, but there is a variable intra-abdominal portion, and in the presence of a hiatal hernia, this portion may be absent. Typical endoscopic measurements from the incisors are from 30 to 40 cm (Figure 22.1). On CT to determine the location, the epicenter of a lower thoracic esophagus/EGJ cancer is below the inferior pulmonary vein. The abdominal esophagus is included in the lower thoracic esophagus. Cancers involving the EGJ that have their epicenter within the proximal 2 cm of the cardia (Siewert types I/II) are to be staged as esophageal cancers. Cancers whose epicenter is more than 2 cm distal from the EGJ, even if the EGJ is involved, will be staged using the stomach cancer TNM and stage groupings (see Chapter 17).
22.1 Anatomy of esophageal cancer primary site by ICD-O-3 topography codes
| Esophageal location | |||||
|---|---|---|---|---|---|
| Anatomic name | Compartment ICD-O-3 | ICD-O-3 | Name | Anatomic boundaries | Typical esophagectomy, cm |
| Cervical | C15.0 | C15.3 | Upper | Hypopharynx to sternal notch | 15 to less than 20 |
| Thoracic | C15.1 | C15.3 | Upper | Sternal notch to azygos vein | 20 to less than 25 |
| C15.4 | Middle | Lower border of azygos vein to inferior pulmonary vein | 25 to less than 30 | ||
| C15.5 | Lower | Lower border of inferior pulmonary vein to EGJ | 30 to less than 40 | ||
| Abdominal | C15.2 | C15.5 | Lower | EGJ to 2 cm below EGJ | 40 to 45 |
| C16.0 | EGJ/cardia | EGJ to 2 cm below EGJ | 40 to 45 | ||
Regional Lymph Nodes
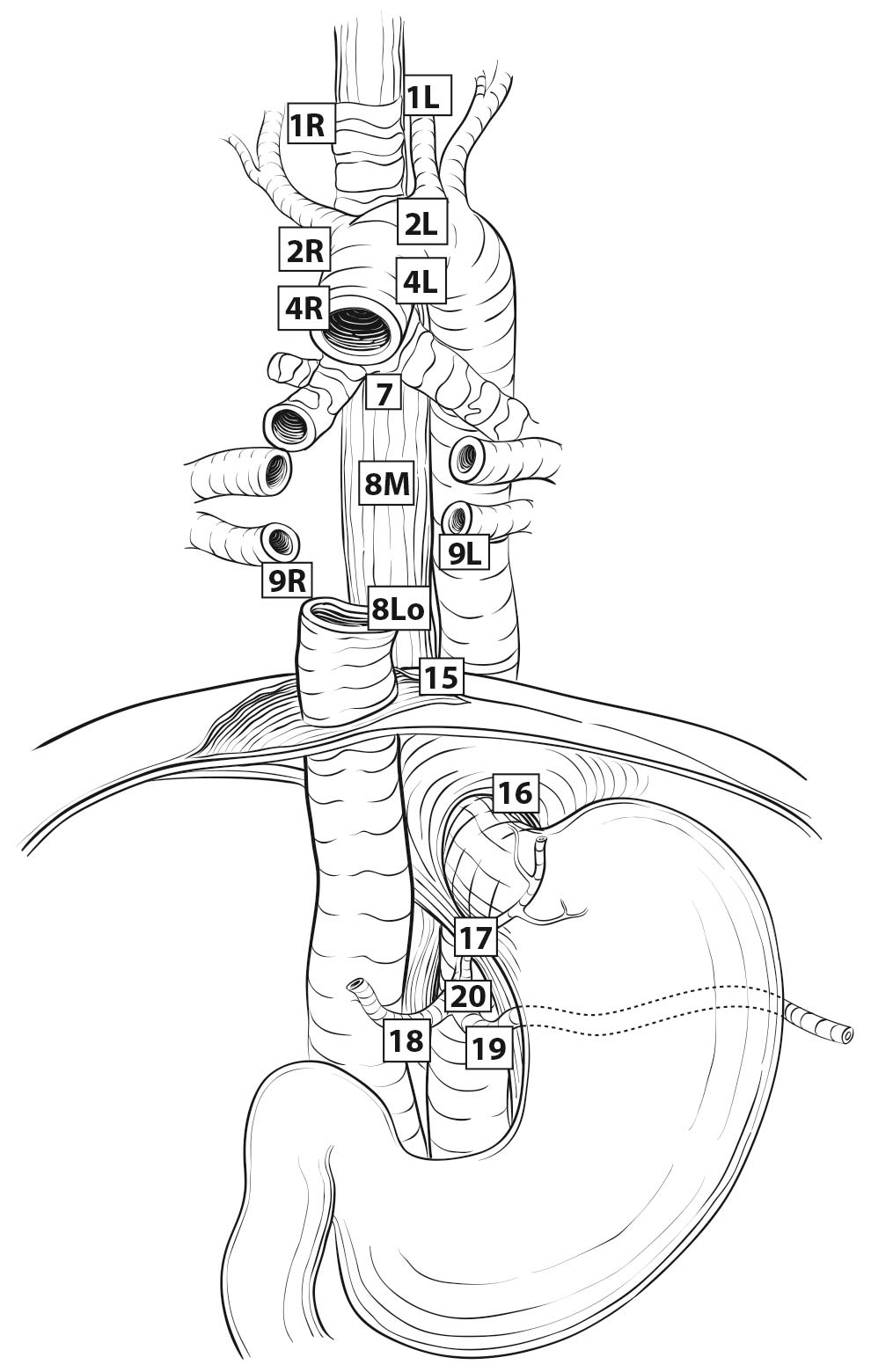
Esophageal lymphatic drainage is intramural and longitudinal. The lymphatic network within the esophagus is concentrated in the submucosa, although lymphatic channels also are present in the lamina propria. This arrangement may permit lymphatic metastases early in the course of the disease from otherwise superficial cancers.10 Lymphatic drainage of the muscularis propria is more limited, but lymphatic channels pierce this layer to drain into regional lymphatic channels and lymph nodes in the periesophageal fat. Up to 43% of autopsy dissections demonstrate direct drainage from the submucosal plexus into the thoracic duct, which facilitates systemic metastases.11-13 The longitudinal nature of the submucosal lymphatic plexus permits lymphatic metastases orthogonal to depth of tumor invasion.14 The implication of the longitudinal nature of lymphatic drainage is that the anatomic site of the cancer and the lymph nodes to which lymphatics drain from that site may not be the same (Figures 22.3_A, 22.3_B, and 22.3_C).
Therefore it follows, and analysis of data supports, that regional lymph nodes for all locations in the esophagus discussed in this chapter extend from periesophageal cervical nodes to celiac nodes (Figures 22.3_A 22.3_B 22.3_Cand 22.4). The nomenclature for thoracic and abdominal regional lymph nodes is listed in Figures 22.3_A, 22.3_B, and 22.3_C. The nomenclature for cervical regional lymph nodes follows that of head and neck chapters (see Chapter 6) and are located in periesophageal levels VI and VII. Lymph nodes in continuity with the esophagus would be considered regional.
The specific regional lymph nodes are as follows:
- Right lower cervical paratracheal nodes: between the supraclavicular paratracheal space and apex of the lung
- Left lower cervical paratracheal nodes: between the supraclavicular paratracheal space and apex of the lung
- Right upper paratracheal nodes: between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung
- Left upper paratracheal nodes: between the top of the aortic arch and apex of the lung
- Right lower paratracheal nodes: between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein
- Left lower paratracheal nodes: between the top of the aortic arch and the carina •Subcarinal nodes: caudal to the carina of the trachea
- Subcarinal nodes: caudal to the carina of the trachea
- Upper thoracic paraesophageal lymph nodes: from the apex of the lung to the tracheal bifurcation
- Middle thoracic paraesophageal lymph nodes: from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein
- Lower thoracic paraesophageal lymph nodes: from the caudal margin of the inferior pulmonary vein to the EGJ
- Pulmonary ligament nodes: within the right inferior pulmonary ligament
- Pulmonary ligament nodes: within the left inferior pulmonary ligament
- Diaphragmatic nodes: lying on the dome of the diaphragm and adjacent to or behind its crura
- Paracardial nodes: immediately adjacent to the gastroesophageal junction
- Left gastric nodes: along the course of the left gastric artery
- Common hepatic nodes: immediately on the proximal common hepatic artery
- Splenic nodes: immediately on the proximal splenic artery
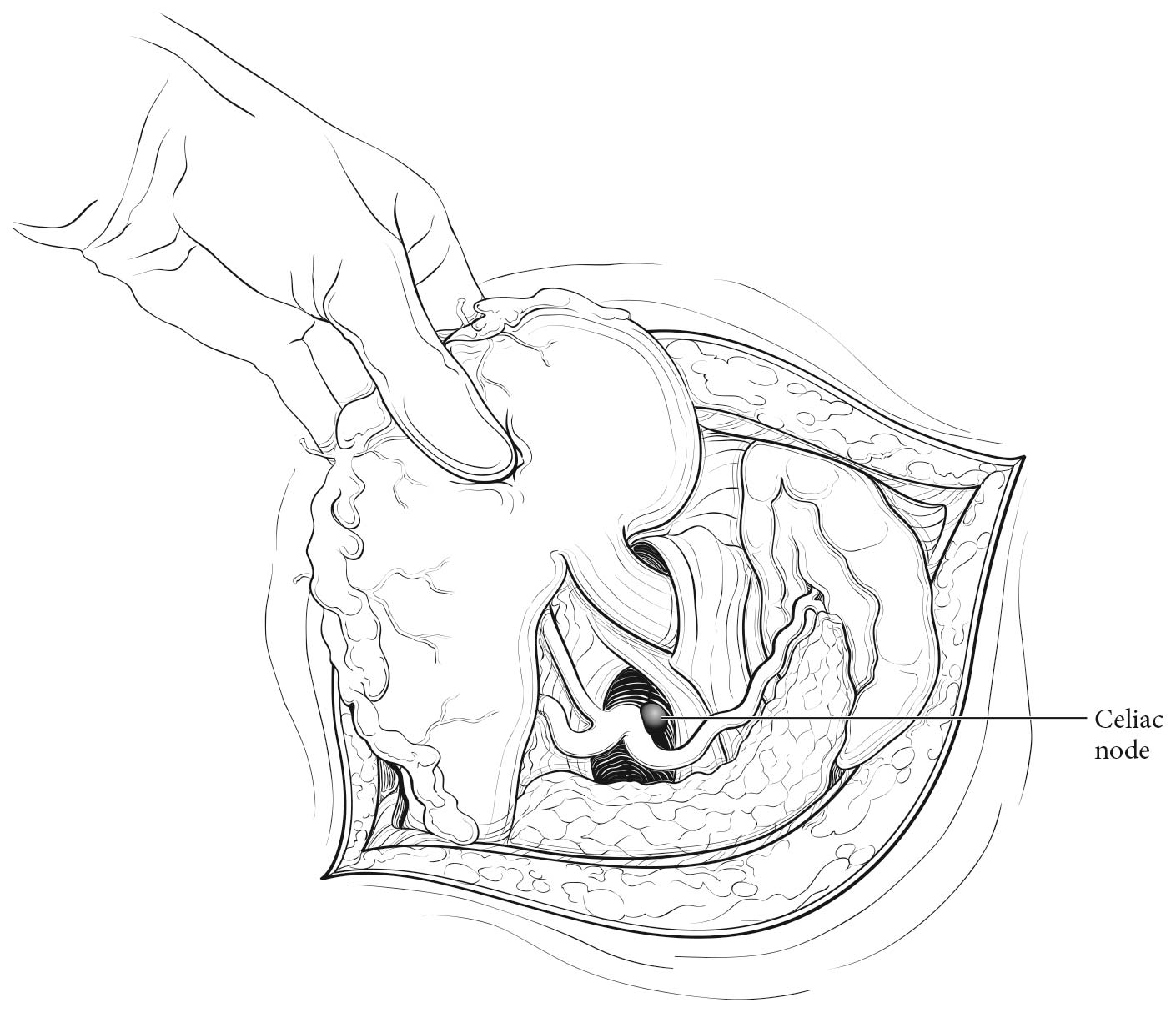
- Celiac nodes: at the base of the celiac artery
- Cervical periesophageal level VI lymph nodes (see Chapter 6)
- Cervical periesophageal level VII lymph nodes (see Chapter 6)
22.3_A (A) Lymph node maps for esophageal cancer. Regional lymph node stations for staging esophageal cancer from left. 1R, Right lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 1L, Left lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 2R, Right upper paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung. 2L, Left upper paratracheal nodes, between the top of the aortic arch and the apex of the lung. 4R, Right lower paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein. 4L, Left lower paratracheal nodes, between the top of the aortic arch and the carina. 7, Subcarinal nodes, caudal to the carina of the trachea. 8U, Upper thoracic paraesophageal lymph nodes, from the apex of the lung to the tracheal bifurcation. 8M, Middle thoracic paraesophageal lymph nodes, from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein. 8Lo, Lower thoracic paraesophageal lymph nodes, from the caudal margin of the inferior pulmonary vein to the EGJ. 9R, Pulmonary ligament nodes, within the right inferior pulmonary ligament. 9L, Pulmonary ligament nodes, within the left inferior pulmonary ligament. 15, Diaphragmatic nodes, lying on the dome of the diaphragm and adjacent to or behind its crura. 16, Paracardial nodes, immediately adjacent to the gastroesophageal junction. 17, Left gastric nodes, along the course of the left gastric artery. 18, Common hepatic nodes, immediately on the proximal common hepatic artery. 19, Splenic nodes, immediately on the proximal splenic artery. 20, Celiac nodes, at the base of the celiac artery.
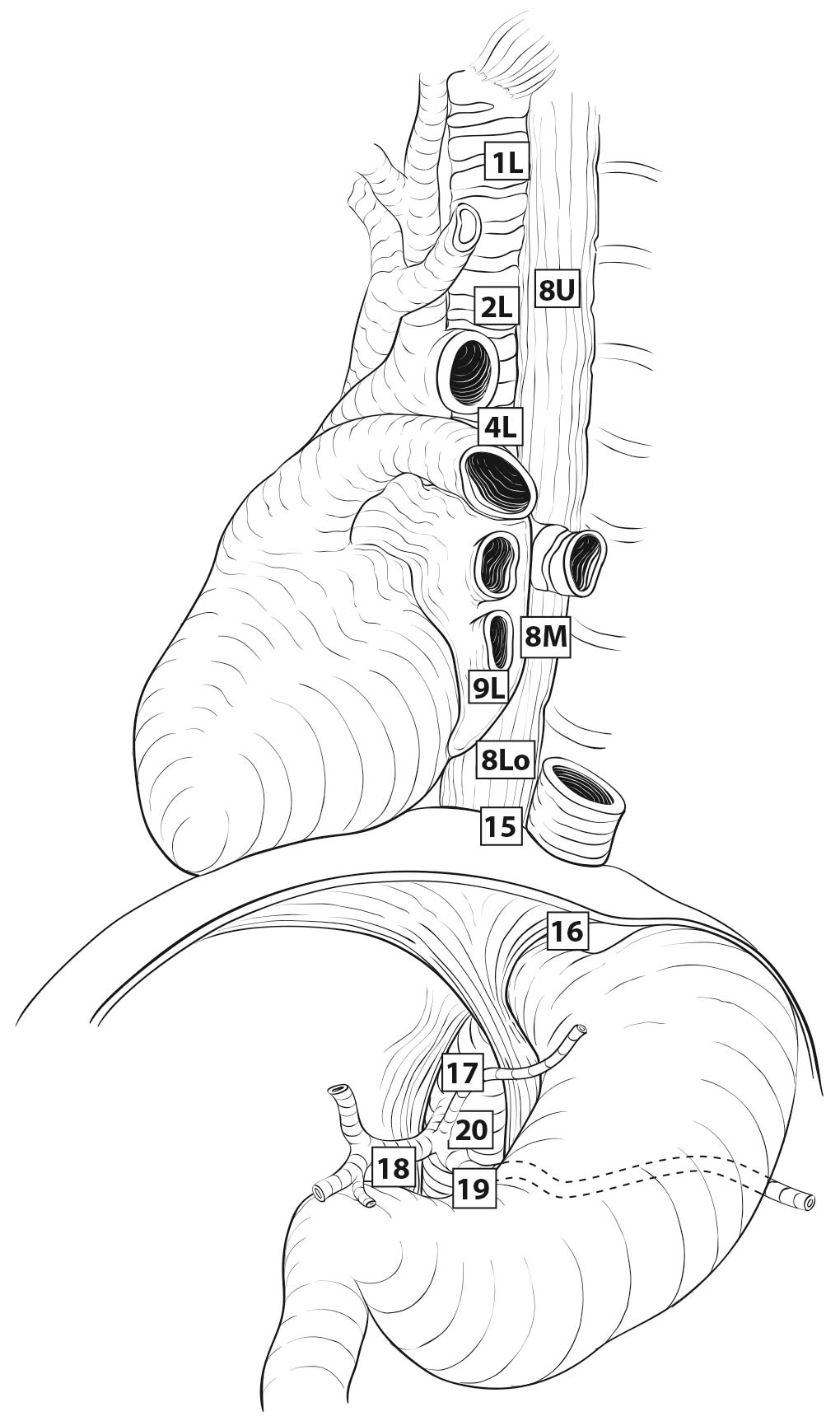
22.3_B (B) Lymph node maps for esophageal cancer. Regional lymph node stations for staging esophageal cancer from right. 1R, Right lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 1L, Left lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 2R, Right upper paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung. 2L, Left upper paratracheal nodes, between the top of the aortic arch and the apex of the lung. 4R, Right lower paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein. 4L, Left lower paratracheal nodes, between the top of the aortic arch and the carina. 7, Subcarinal nodes, caudal to the carina of the trachea. 8U, Upper thoracic paraesophageal lymph nodes, from the apex of the lung to the tracheal bifurcation. 8M, Middle thoracic paraesophageal lymph nodes, from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein. 8Lo, Lower thoracic paraesophageal lymph nodes, from the caudal margin of the inferior pulmonary vein to the EGJ. 9R, Pulmonary ligament nodes, within the right inferior pulmonary ligament. 9L, Pulmonary ligament nodes, within the left inferior pulmonary ligament. 15, Diaphragmatic nodes, lying on the dome of the diaphragm and adjacent to or behind its crura. 16, Paracardial nodes, immediately adjacent to the gastroesophageal junction. 17, Left gastric nodes, along the course of the left gastric artery. 18, Common hepatic nodes, immediately on the proximal common hepatic artery. 19, Splenic nodes, immediately on the proximal splenic artery. 20, Celiac nodes, at the base of the celiac artery.
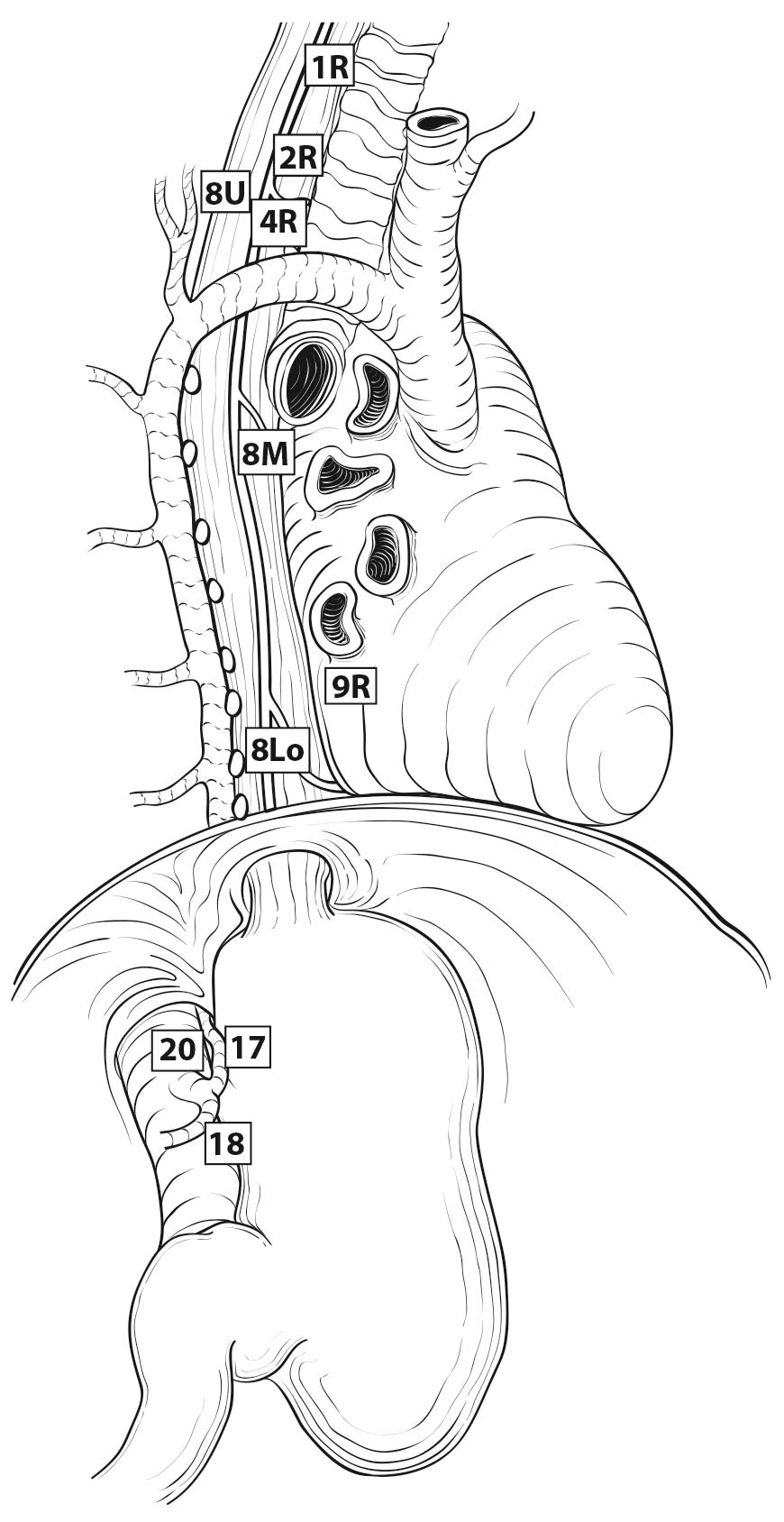
22.3_C C) Lymph node maps for esophageal cancer. Regional lymph node stations for staging esophageal cancer, anterior view. 1R, Right lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 1L, Left lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 2R, Right upper paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung. 2L, Left upper paratracheal nodes, between the top of the aortic arch and the apex of the lung. 4R, Right lower paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein. 4L, Left lower paratracheal nodes, between the top of the aortic arch and the carina. 7, Subcarinal nodes, caudal to the carina of the trachea. 8U, Upper thoracic paraesophageal lymph nodes, from the apex of the lung to the tracheal bifurcation. 8M, Middle thoracic paraesophageal lymph nodes, from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein. 8Lo, Lower thoracic paraesophageal lymph nodes, from the caudal margin of the inferior pulmonary vein to the EGJ. 9R, Pulmonary ligament nodes, within the right inferior pulmonary ligament. 9L, Pulmonary ligament nodes, within the left inferior pulmonary ligament. 15, Diaphragmatic nodes, lying on the dome of the diaphragm and adjacent to or behind its crura. 16, Paracardial nodes, immediately adjacent to the gastroesophageal junction. 17, Left gastric nodes, along the course of the left gastric artery. 18, Common hepatic nodes, immediately on the proximal common hepatic artery. 19, Splenic nodes, immediately on the proximal splenic artery. 20, Celiac nodes, at the base of the celiac artery.
T
Malignant cells confined to the esophageal epithelium are categorized as Tis (high-grade dysplasia). Cancers confined to the mucosa are T1a (intramucosal), and those that invade beyond, but are confined to the submucosa, are T1b (submucosal). Cancers confined to the muscularis propria are T2. Cancers invading the adventitia are T3. Cancers invading adjacent structures are T4, which are subcategorized into T4a and T4b. See Figure 22.5.
N
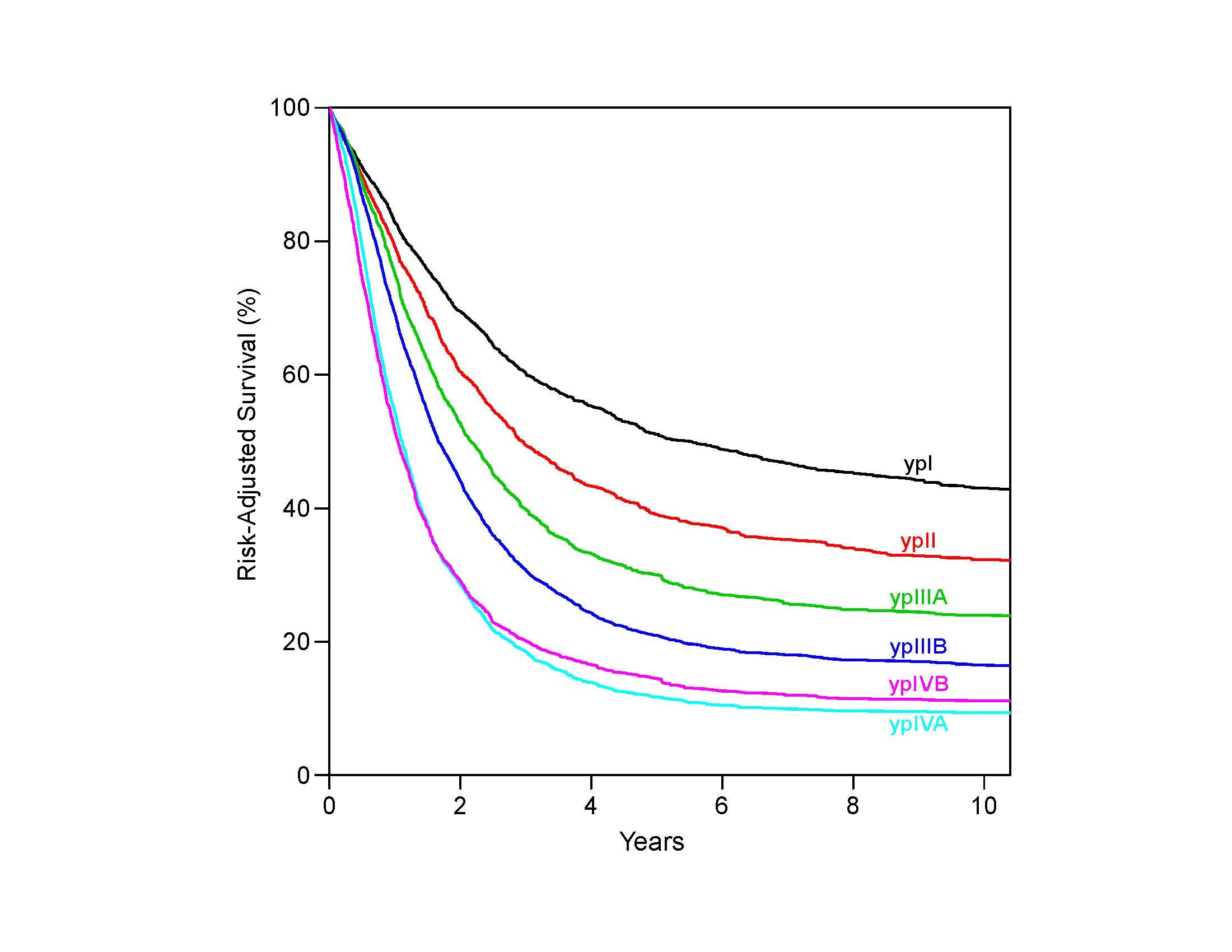
The data on which this chapter is based demonstrate that the total number of lymph nodes containing metastases (positive nodes) is an important prognostic factor. In classifying N, the data support convenient coarse groupings of number of positive nodes (zero, one to two, three to six, seven or more). These groups have been designated N1 (one to two), N2 (three to six), and N3 (seven or more) (Figure 22.5). Nevertheless, there are no sharp cut points; rather, each additional positive node reduces survival. Clinical determination of the number of positive lymph nodes is possible and correlates with survival.15-17
M
If there is no evidence of metastasis to distant sites, the category is M0. If metastases to distant sites are evident, these are categorized as M1 (Figure 22.5).
Classifications
Staging recommendations presented in this chapter for adenocarcinoma of the esophagus and EGJ apply to clinical staging (cTNM; newly diagnosed, not yet treated patients), pathological staging (pTNM) for patients directly undergoing resection without prior treatment, and patients who have received preoperative therapy (ypTNM).
Clinical Classification
Clinical assessment begins with a patient's history and physical examination. The recent onset of dysphagia and weight loss often heralds at least locally advanced disease. Abnormal physical findings suggesting distant metastasis, such as palpable lymphadenopathy or subcutaneous masses, should prompt immediate definition of the cause via imaging, aspiration cytology, biopsy, or other methods.
Imaging and endoscopy currently are critical components of clinical staging. This section describes current recommendations for studies to define T, N, and M. Blood-based assays and tumor genomics analysis so far have not identified validated biomarkers to inform staging.
Imaging
Given the disparity in outcomes when comparing esophageal cTNM with pTNM staging, there clearly is a need for more accurate and precise clinical staging modalities. It is important for physicians to clearly indicate in the medical record the modalities used to determine clinical stage (e.g., endoscopy with or without biopsy, endoscopic resection, CT, fluorine-18 fluoro-2-deoxy-D-glucose [FDG] positron emission tomography [PET]/CT, endoscopic ultrasound [EUS] with or without fine-needle aspiration [FNA]). These data will inform future clinical staging systems.
CT of the chest and abdomen with oral and intravenous contrast frequently is the initial imaging modality used to determine the proximity of the tumor to other structures, as well as the cN and cM categories. PET/CT with FDG is used to further refine cN category away from the primary tumor, and is more sensitive than CT for determining cM category.18-26 Some of these studies suggest that FDG PET/CT may also be useful in estimating the extent of gastric tumor extension for lower EGJ tumors, especially in obstructing tumors of the esophagus (Figure 22.1).
CT of the chest and abdomen with intravenous and oral contrast and FDG PET/CT imaging may be used to describe the primary cancer in terms of location in the cervical, upper thoracic, middle thoracic, lower thoracic, or abdominal esophagus, as well as its orientation to other structures. Determination of locoregional involvement with regard to adjacent structures is important in treatment planning. However, CT of the chest and abdomen and FDG PET/CT have a limited role in determining primary tumor category (cT). The inability to differentiate between cT1, cT2, and cT3 and invasion of adjacent structures (cT4) is a major limitation in the use of CT for the primary tumor category (cT). Additionally, although the intensity of FDG uptake and cT category are positively related, this association is weak.18,27,28
CT of the chest and abdomen with intravenous and oral contrast and FDG PET/CT imaging may be used to describe locoregional (cN) lymph nodes. Unfortunately, CT and FDG PET/CT imaging are not optimal for detecting locoregional nodal metastasis because of their low accuracy.18,19,21-23,26 In clinical practice, locoregional nodes generally are suspicious for tumor involvement when round and/or greater than 10 mm in short axis diameter. The portocaval lymph node, however, is an exception to these criteria. This lymph node has an elongated shape with a long transverse diameter and small anterior posterior diameter, and relying on measurement alone would result in frequent false positive interpretations. Additionally, the diagnostic benefit of FDG PET/CT is especially limited in patients with an early T category (pT1) because of the low prevalence of nodal and distant metastases and the high rate of false positive PET findings.27,29 Because the criteria for cN category have not been defined rigorously in peer-reviewed literature, the current cN category requires evaluation of the size, shape, and number of abnormal lymph nodes in determining the cN category by imaging. As we make an effort to make clinical stage more accurate, obtaining histologic samples through various endoscopic techniques (endobronchial ultrasound, EUS-FNA) also should be considered.
CT of the chest and abdomen with intravenous and oral contrast and FDG PET/CT imaging are useful in detecting distant metastasis (cM). The addition of FDG PET/CT imaging to conventional clinical staging improves the detection of distant metastases missed or not visualized on CT of the chest and abdomen. However, a potential pitfall is the poor detection of hepatic metastases when the CT component of the FDG PET/CT is performed without intravenous contrast. An additional pitfall is the high rate of false positive PET findings that may result in unnecessary additional investigations.23,25-27,29,30 Furthermore, the diagnostic benefit of performing FDG PET/CT may be limited if comprehensive conventional staging, including CT of the chest, abdomen and pelvis; EUS; and sonography of the neck, is performed.
Recent improvements in magnetic resonance (MR) imaging techniques have resulted in better imaging quality and improved determination of cT and cN categories.31-33 In addition, whole-body MR imaging with or without diffusion weighting may have a role in cM categorization. However, a current limitation is that because MR imaging is not commonly performed in the staging of patients with esophageal cancer, the studies indicating its utility in staging are small, and the ultimate role of MR imaging in staging is uncertain.
Endoscopy (cT, cN, c/pM, G, L)
Esophagoscopy with multiple biopsies provides information on cancer location (L) and tissue to determine the cell type and histologic grade (G) of the tumor. Location of the primary tumor in relation to the EGJ should always be documented for purposes of appropriate staging and therapy. The presence of skip lesions (multiple discrete lesions) should be recorded and included in the overall length of the tumor. This requires the suffix m: T(m).
The clinical assessment of depth of tumor invasion and nodal involvement, as well as some limited areas of distant disease, may be facilitated by the use of EUS or EUS-FNA. Esophageal staging is best performed with the use of commercially available ultrasound endoscopes with multifrequency (5-, 7.5-, 10-, and 12-MHz) radial transducers.34
Sonographic evaluation is performed as the instrument is withdrawn starting at the pylorus. Orienting the images in an anteroposterior axis enables careful assessment of anatomic landmarks to permit correlation with the location of the tumor, lymph nodes, and surrounding organs. The individual layers of the gastrointestinal wall are visualized throughout the examination, to correlate the extent of the tumor relative to the alternating bright and dark layers seen on ultrasound. On the basis of in vitro studies, the first two layers (bright and dark starting at the lumen) correspond to the acoustical interface and mucosa, the third (bright) layer corresponds to the submucosa, the fourth (dark) layer to the muscularis propria, and the fifth (bright) layer to the adventitia.35 Alterations in thickness of individual layers are identified, permitting an estimate of depth of tumor invasion (cT).
The presence of a mass in the esophagus usually is diagnosed as a hypoechoic or dark thickening in one or more layers, or loss of the usual layer pattern.34,35 The first bright layer, which represents a transition echo layer, rarely is lost or thickened. Thickening of the second layer, or the inner dark layer, suggests a cT1 tumor. Although at higher EUS frequencies of 10 or 12 MHz one should be able to distinguish tumors limited to the mucosa (cT1a) from those extending into the submucosa (cT1b), most studies have shown poor accuracy.36-39 A dark thickening extending from the second to the third layer (mucosa and submucosa) but not reaching the fourth layer (muscularis propria) is evidence of a T1b tumor. A dark thickening extending to the fourth layer with a smooth outer border is associated with a cT2 tumor.
Suspicious nodules or lesions known to be malignant that are identified on endoscopy as potentially superficial should be excised by endoscopic resection to provide the best available determination of tumor depth in early carcinomas. Ultimately, a cancer that is completely removed by endoscopic resection (negative deep margin designated by a pathologist) should be designated as pT. The final stage designation of a patient who has undergone endoscopic resection followed by esophagectomy must take into account the conglomerate pathology results, using the deepest point of invasion for the final pT category.
Complete loss of all the layers, associated with an irregular outer surface, indicates penetration beyond the muscularis propria, consistent with a cT3 tumor in the esophagus. If the dark thickening extends to the pleura, pericardium, azygos vein, diaphragm, or peritoneum, the tumor is categorized as cT4a. Extension through the muscularis propria with loss of the echogenic stripe separating the esophagus from surrounding structures, such as the aorta, heart, lung parenchyma, or other adjacent structure, indicates a cT4b tumor.
The lymphatic drainage areas routinely investigated are both regional and nonregional (cN, cM), including the peritumoral, paratracheal, subcarinal, crural, celiac axis, splenic vein, portacaval, and gastrohepatic ligament areas. The presence of hypoechoic, rounded, sharply demarcated structures in these areas is considered diagnostic of malignant lymph nodes.34,36,37 Histologic confirmation of nodal disease (cN) by EUS-FNA is strongly encouraged.39,40 Since the 7th Edition of AJCC staging, clinical nodal staging in these areas has required documentation of the number and location of suspicious nodes. The appropriate nodal staging by EUS should include reporting of the number of suspicious nodes seen during the examination, followed by interpretation of the categorization according to AJCC N criteria: no suspicious nodes, N0; one or two suspicious nodes, N1; three to six suspicious nodes, N2; and seven or more suspicious nodes, N3.
Parts of the liver are readily seen with EUS with the endoscope positioned in the antrum and along the lesser curvature and cardia, permitting the identification of liver metastases (M1). Similarly, the presence of ascites adjacent to the stomach raises suspicion for peritoneal metastases, if other causes of ascites are ruled out.41,42 This, however, has not been shown to be a reliable indicator of M1 disease. If the site of distant metastases is seen on imaging or on EUS without histologic confirmation, the metastases should be considered clinically determined (cM1). If a biopsy is performed (strongly encouraged) and there is pathological confirmation of cancer, then it is assigned pM1 for the clinical classification.43
Pathological Classification
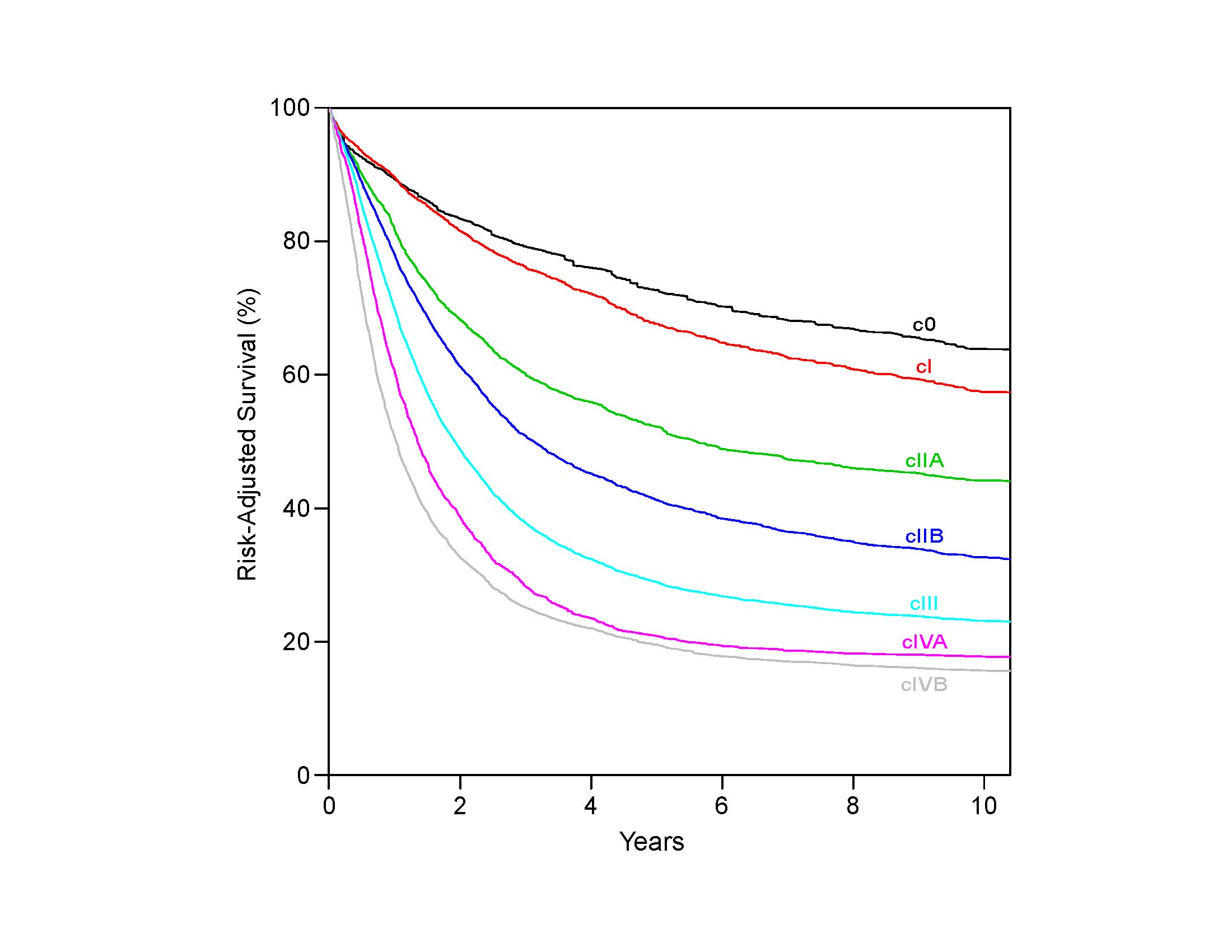
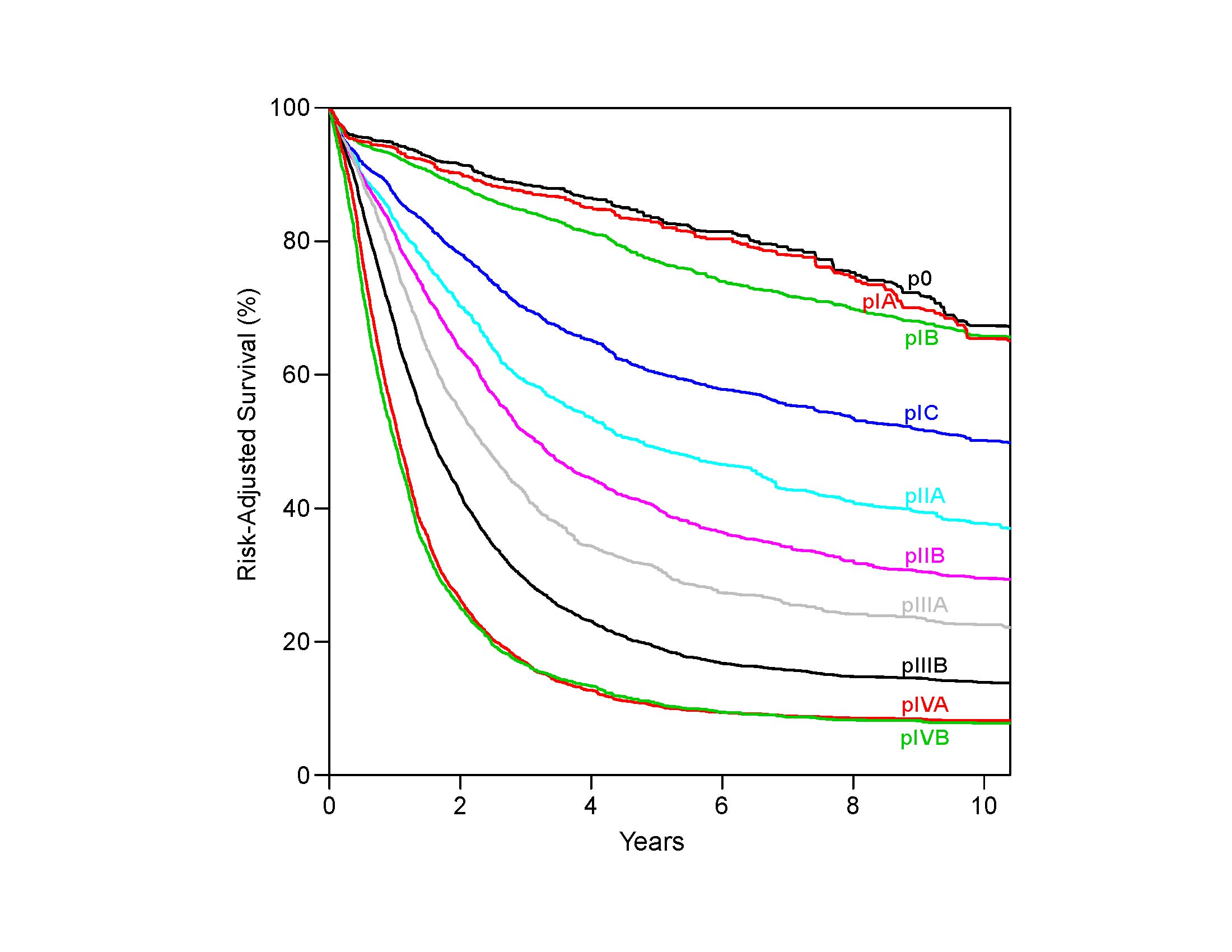
Comparing the survival of patients receiving surgery alone (pTNM) with that of patients receiving neoadjuvant therapy (ypTNM) with equivalent pathological classifications, it is evident that prognostic implications for neoadjuvant stage classifications differ from those of equivalent pathological stage classifications (pTNM).2,4-6 Survival of node-negative patients receiving neoadjuvant therapy (ypN0) is worse than that of equivalently pathologically staged patients undergoing esophagectomy alone (pN0); the prognosis of node-positive patients receiving neoadjuvant therapy (ypN+) is either worse or no better than that of equivalently staged patients receiving esophagectomy alone (pN+). Therefore, separate stage groupings for p and yp groupings are needed to stage patients more accurately within each treatment algorithm.
Accurate pathological staging requires careful examination of the gross specimen in terms of tumor size, shape, configuration, location, distance from margins (proximal, distal, and radial/circumferential), and nodal dissection. Amalgamation with clinical data is critical for pretreatment length or for final depth determination in patients who have undergone previous endoscopic resection. Pretreatment clinical M category (cM) would be included in the definition of ypTNM unless upstaged from cM0 to pM1 after resection (ypTypNcM).
Adjacent Structures
In close proximity to the esophagus lie the pleura, peritoneum, pericardium, azygos vein, and diaphragm. Cancers invading these structures are subcategorized as T4a. The aorta, arch vessels, airway, and vertebral body also are nearby, but cancers invading these structures are subcategorized as T4b.
Regional Lymph Node Assessment
Data demonstrate that in general, the more lymph nodes resected, the better the survival, which may be the result of either improved N categorization or a therapeutic effect of lymphadenectomy. Based on worldwide data, the adequacy of lymphadenectomy depends on T categorization. For pT1, approximately 10 nodes must be resected to maximize survival; for pT2, 20 nodes; and for pT3 or pT4, 30 nodes or more.44 Based on different data and analysis methods that focus on maximizing sensitivity, others have suggested that an adequate lymphadenectomy requires resecting 12 to 23 nodes.45,46 However, to determine pN category adequately, paradoxically more nodes must be resected for early-stage cancers than for advanced-stage cancers.47 Overall, it is desirable to resect as many regional lymph nodes as possible, balancing the extent of lymph node resection necessary to accurately determine pN and maximize survival without unnecessarily increasing the morbidity of radical lymphadenectomy.
Optimal lymph node yield and staging depend on the amount of nodal tissue resected by the surgeon as well as specimen handling by pathology personnel. The periesophageal soft tissue should be dissected thoroughly to maximize the lymph node yield. In cases in which lymph node tissue is submitted so that nodes may be individually counted, the number of lymph nodes should be documented in the pathology report. In cases in which the nodal specimens are received in multiple fragments, accurate lymph node count may not be possible, and this finding should be documented. However, in such cases, the surgeon should note the number of lymph nodes submitted in the fragmented specimen.
In patients who have received neoadjuvant therapy, lymph nodes may undergo atrophy and may be difficult to recognize macroscopically. Extent of lymphadenectomy may not be as related to survival as in pTNM.45 In these cases, histologic assessment of most of the periesophageal soft tissue is helpful to retrieve grossly impalpable lymph nodes.
Following neoadjuvant treatment, the lymph node parenchyma shows fibrosis, lymphoid depletion, and acellular mucin lakes. Lymph nodes with these changes, and without any viable tumor cells, should be considered negative for metastasis. Immunohistochemical stains, such as cytokeratin AE1/AE3, may be used to confirm the presence of rare residual tumor cells. However, as false positive results may occur, they should be interpreted in conjunction with morphologic findings.
Distant Metastasis
The categorization of distant metastasis for pathological staging may be cM0, cM1, or pM1. Extensive imaging is not required to assign cM0. Distant metastasis identified on imaging or during surgery but not biopsied is assigned cM1. Histologic evidence of distant metastasis is categorized as pM1.
In postneoadjuvant therapy staging (yp), the M category is identified during clinical staging and is not changed based on the response to therapy, unless upstaged from cM0 to pM1.
- Clinical staging modalities (endoscopy and biopsy, EUS, EUS-FNA, CT, PET/CT)
- Tumor length
- Depth of invasion
- Number of nodes involved, clinical
- Number of nodes involved, pathological
- Location of nodal disease, clinical
- Location of nodal disease, pathological
- Sites of metastasis, if applicable
- Presence of skip lesions: T(m)
- Perineural invasion
- LVI (lymphatic, vascular, both)
- Extranodal extension
- HER2 status (positive or negative)
- Type of surgery
- Chemotherapy
- Chemoradiation theraypy (for ypTNM)
- Surgical margin (negative, microscopic, macroscopic)