The pancreas is a coarsely lobulated gland that lies transversely across the posterior abdomen and extends from the duodenum to the splenic hilum. The organ is divided into a head with an uncinate process, a neck, a body, and a tail (Figure 36.1). The anterior aspect of the body of the pancreas is in direct contact with the posterior wall of the stomach; posteriorly, the pancreas extends to the inferior vena cava, superior mesenteric vein, splenic vein, and left kidney. The uncinate process is part of the pancreatic head and may extend posterior to the superior mesenteric vein and artery. The pancreatic head accounts for 60-70% of pancreatic adenocarcinomas, whereas 20-25% arise in the body and tail and 10-20% diffusely involve the pancreas.1
36.1 Tumors of the head of the pancreas are those arising to the right of the superior mesenteric-portal vein confluence. Tumors of the body of the pancreas are those arising between the left border of the superior mesenteric vein and the left border of the aorta. Tumors of the tail of the pancreas are those arising between the left border of the aorta and the hilum of the spleen.
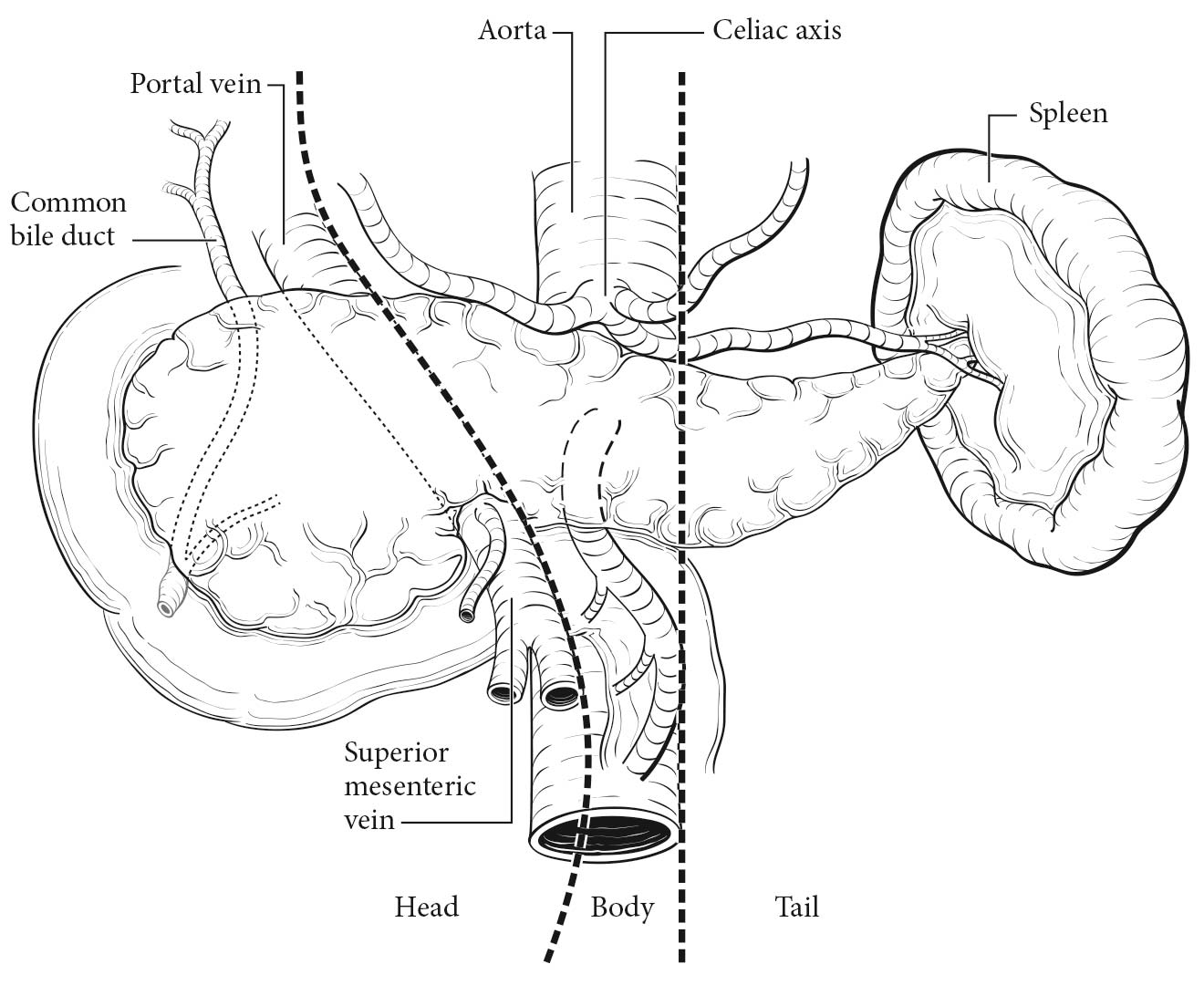
A rich lymphatic network surrounds the pancreas, and accurate tumor staging requires resection of peripancreatic lymph nodes for pathological assessment. The standard regional lymph node basins and soft tissues resected for tumors located in the head and neck of the pancreas include lymph nodes along the common bile duct, common hepatic artery, portal vein, pyloric, posterior and anterior pancreatoduodenal arcades, and along the superior mesenteric vein and right lateral wall of the superior mesenteric artery. For cancers located in the body and tail, regional lymph node basins include lymph nodes along the common hepatic artery, celiac axis, splenic artery, and splenic hilum. Tumor involvement of other nodal groups is considered distant metastasis.
Information necessary for the clinical staging of pancreatic cancer may be obtained from physical examination and three-dimensional radiographic imaging studies, which include triphasic, contrast-enhanced multislice computed tomography (CT) or magnetic resonance (MR) imaging. Endoscopic ultrasonography (if done by experienced gastroenterologists) also provides information helpful for clinical staging and is the procedure of choice for performing fine-needle aspiration (FNA) biopsy of the pancreas. The standard radiographic assessment of resectability includes evaluation for peritoneal or hepatic metastases; the patency of the superior mesenteric vein and portal vein and the relationship of these vessels and their tributaries to the tumor; and the relationship of the tumor to the superior mesenteric artery, celiac axis, and hepatic artery. If the appropriate clinical and radiographic findings are present, preoperative biopsy is not necessary before resection, but a core biopsy or an endoscopic ultrasound (EUS)-guided fine-needle aspiration biopsy specimen should be obtained to confirm the diagnosis for patients undergoing neoadjuvant therapy. Serum IgG4 levels may be helpful in identifying autoimmune pancreatitis if a tissue diagnosis has not been obtained.
Laparoscopy may be performed in patients believed to have localized, potentially resectable tumors to exclude peritoneal metastases and small metastases on the surface of the liver. Laparoscopy will reveal small (less than 1 cm) peritoneal or liver metastases and upstage (to Stage IV) approximately 10% of patients with tumors in the pancreatic head, and probably a greater percentage of patients with tumors in the body and tail. A significantly elevated preoperative cancer antigen (CA) 19-9 level (greater than 250 U/mL) increases the yield of staging laparoscopy.
Pancreatic cancers that are locally advanced have a low likelihood of resectability and a high chance of incomplete removal if resected. This scenario has given rise to the term borderline resectable tumor, which has been defined by the Americas Hepato-Pancreato-Biliary Association/Society of Surgical Oncology/Society for Surgery of the Alimentary Tract, the International Study Group of Pancreatic Surgery (ISGPS), and the National Comprehensive Cancer Network (NCCN).2,3 The superior mesenteric artery may have tumor abutment up to but not more than 180o of the circumference of the vessel and may have short segment involvement of the hepatic artery that does not extend onto the celiac axis. Venous involvement of the superior mesenteric vein/portal vein is defined as tumor abutment with or without impingement and narrowing of the lumen or short-segment venous occlusion resulting from either tumor thrombus or encasement but with suitable vessel proximal and distal to the area of vessel involvement. In general, these locally advanced cancers should be treated as part of a clinical trial or receive neoadjuvant therapy.
Imaging
Cross-sectional imaging, either contrast-enhanced, multiphasic, thin-section MR imaging or CT, typically is the preferred examination for assessing the stage of pancreatic cancers, ampullary tumors, and distal common bile duct tumors and should be performed before any interventions (e.g., biopsy, stent placement). The choice of MR imaging or CT should be based on the imaging equipment available, the expertise of the radiologists performing and interpreting the studies, and whether there are confounding issues, such as allergies to intravenous contrast or renal insufficiency (in the latter case, unenhanced MR imaging is preferred to unenhanced CT because of MR imaging's superior soft tissue contrast). Imaging should be performed before interventions (e.g. stent placement, biopsy) to avoid the effects of potential postprocedure pancreatitis interfering with staging assessments.
If intravenous contrast is used, dynamic imaging (MR imaging or CT) should be performed both during the phase of peak pancreatic enhancement (“pancreatic parenchymal” or “late arterial” phase), to enhance the conspicuity of tumor against the background pancreas (regardless of ampullary, pancreatic, or distal biliary origin), and during the portal venous phase of liver enhancement (peak liver enhancement) and when veins are fully opacified, to judge extrapancreatic extent of tumor, involvement of vasculature, and the possibility of liver metastases, as liver metastases from these tumors typically are hypodense against uninvolved liver. Thin-section imaging (2-3 mm for CT, thicker but as thin as reasonably possible for MR imaging) is particularly important for judging vascular involvement and to assess for potential small sites of metastatic disease. In the setting of preoperative therapy, such technique is important not only at baseline but also following therapy to determine whether patients are still surgical candidates and to follow up borderline suspicious findings.
Endoscopic ultrasound may be used next after CT/MR imaging for problem-solving, given that it is a relatively invasive technique and has limited utility for assessing for distant disease such as liver metastases, peritoneal implants, or adenopathy outside the surgical field. However, the ability of EUS to detect small tumors and guide biopsy is particularly helpful for tumors that may appear isodense to, and therefore indistinguishable from, background pancreas on CT and/or MR imaging. EUS and EUS-FNA also should be performed before ERCP, as pancreatitis may degrade the ability of EUS to visualize tumor and the placement of stents eliminates the ability to identify sites of duct cutoff that may be useful in guiding biopsies. ERCP subsequently may be helpful in the setting of duct abnormalities, both for treatment (stent placement) and for diagnosis (brushings).4-21
TNM Categories of Staging by Imaging
The T category is assessed by measuring the largest diameter of the tumor in the axial plane, whether by CT or by MR imaging. With regard to MR imaging, the measurement should be made on the sequence that best delineates the tumor. Note should be made if there is suspicion that pancreatitis may be present, which may alter the apparent size of the tumor.
The relationship of tumor to relevant vessels should be reported, including its relationship to arteries such as the superior mesenteric, celiac, splenic, and common hepatic arteries, as well as the aorta if the tumor extends sufficiently posteriorly into the retroperitoneum. The tumor's relationship to relevant veins includes the portal vein, splenic vein, splenoportal confluence, and superior mesenteric vein, as well as branch vessels such as the gastrocolic trunk, first jejunal vein, and ileocolic branches. The goal is to delineate tumor extent sufficiently to provide useful information for potential en bloc resection with vascular graft placement.
The relationship of tumor to vessels should be described using terms commonly understood by the clinical community, such as degrees of circumferential involvement and the terms abutment (i.e., less than or equal to 180° of involvement of a given vessel by tumor) and encasement (i.e., greater than 180° of circumferential vessel involvement by tumor). Multiplanar reconstructions for CT and direct multiplanar imaging for MR imaging may be particularly helpful in visualizing the circumferential relationship of tumor to relevant vasculature. It also is important to describe the relationship of tumor to adjacent structures, such as the stomach, spleen, colon, small bowel, and adrenal glands.
Assessment of N category (nodal) status is a challenge for all imaging modalities, because all are limited with regard to detection of microscopic metastatic disease to nodes. Nevertheless, it is important to fully identify the location of visibly suspicious nodes. Nodes are considered suspicious for metastatic involvement if they are greater than 1 cm in short axis or have abnormal morphology (e.g., are rounded, are hypodense or heterogeneous, have irregular margins, involve adjacent vessels or structures).
The most common sites of metastatic disease include the liver, peritoneum, lung, and bone, with metastases to the latter two sites usually occurring late in the disease. Evaluations for potential metastases are best done with contrast-enhanced CT and MR imaging; MR imaging likely provides superior capability for assessing potential liver metastases and involvement of bone, whereas CT is better for evaluating potential lung metastases.
Suggested Radiology Report Format
With the development of neoadjuvant therapy and the category of borderline resectable disease, it is particularly important that radiology reports use commonly understood terminology and that borderline suspicious findings, whether of tumor involvement of vasculature or of potential metastatic disease, such as to the liver, be noted so that they can be monitored on follow-up.
Details of the radiology report should include descriptions of:
- Primary tumor: location, size, characterization (enhancement pattern, e.g., hypodense, hyperdense, cystic, or mixed), and effect on ducts (common bile duct and main pancreatic duct). It also should be noted whether there are findings suspicious for superimposed acute pancreatitis, which may distort findings relevant to staging, or chronic pancreatitis/autoimmune pancreatitis, because these diseases may closely mimic malignancy and may be associated with duct strictures.
- Local extent: the relationship of tumor, with reference to degrees of circumferential involvement using commonly understood terms such as abutment and encasement, and occlusion with regard to adjacent arterial structures (celiac, superior mesenteric, hepatic, and splenic arteries and the aorta) and venous structures (portal, splenic, and superior mesenteric veins, and if relevant, inferior vena cava).
- It also should be noted how much of the vascular involvement is related to solid tumor versus stranding, and whether vessel involvement is related to direct involvement by tumor or is distinctly separate from the prior tumor.
- Other descriptors that should be reported include narrowing of the vasculature, vascular thrombi, and potentially, the length of involvement by tumor.
- In the case of borderline resectable disease and tumor involvement of the common hepatic artery, it should be noted whether there is sparing of the origin of the common hepatic artery from the celiac, as well as the length of that sparing, because vascular grafting may be considered.
- In the case of borderline resectable disease and tumor involvement of the common hepatic artery, it should be noted whether there is sparing of the origin of the common hepatic artery from the celiac, as well as the length of that sparing, because vascular grafting may be considered.
- The presence of enlarged collaterals or varices should be noted.
- The involvement of branch vessels such as the gastrocolic, first jejunal, and ileocolic branches of the superior mesenteric vein should be noted. These findings are particularly relevant in planning the extent and feasibility of venous vascular grafts.
- Relevant arterial variants: This information is particularly important with regard to hepatic arterial variants, such as those arising from the superior mesenteric artery, and to the nature of the variant (e.g., accessory right hepatic vs. common hepatic artery arising from the superior mesenteric artery). Confounding factors, such as narrowing of the celiac origin by arcuate ligament syndrome or atherosclerotic disease of the celiac and superior mesenteric arteries, and their effects on adjacent vasculature also are important for treatment planning.
- Lymph node involvement: Suspicious nodes should be documented, particularly if they are greater than 1 cm in short axis or morphologically abnormal (e.g., are rounded, are hypodense/heterogeneous/necrotic, have irregular margins).
- Distant spread: Evaluation should include the liver, peritoneum (including whether ascites is present or absent), bone, and lung. Note should be taken of indeterminate lesions, particularly if they are too small to characterize, because they may be monitored on follow-up imaging to assess for growth or resolution.
- Ascites should be noted because it may indicate peritoneal metastases; however, it should be addressed in the context of whether there are confounding secondary causes of ascites, such as superior mesenteric vein or portal vein narrowing or occlusion.
- Unexpected but notable other findings relevant to management should be noted and described as well.
Partial resection (pancreaticoduodenectomy or distal pancreatectomy) or complete resection of the pancreas, including the tumor and associated regional lymph nodes, provides the information necessary for pathological staging. In pancreaticoduodenectomy specimens, the bile duct, pancreatic parenchymal, uncinate, proximal (duodenal or gastric), and distal duodenal margins should be evaluated. The pancreatic parenchymal margin is also referred to as the pancreatic duct margin, pancreatic neck margin, and distal pancreatic resection margin. The uncinate margin has also been termed the superior mesenteric artery margin, retroperitoneal margin, mesopancreatic margin, posterior-inferior margin, deep margin, and radial margin. All margins except the pancreatic parenchymal margin should be assessed in total pancreatectomy specimens. The College of American Pathologists (CAP) Checklist for Exocrine Pancreatic Tumors is recommended as a guide for the pathological evaluation of pancreatic resection specimens (www.cap.org).
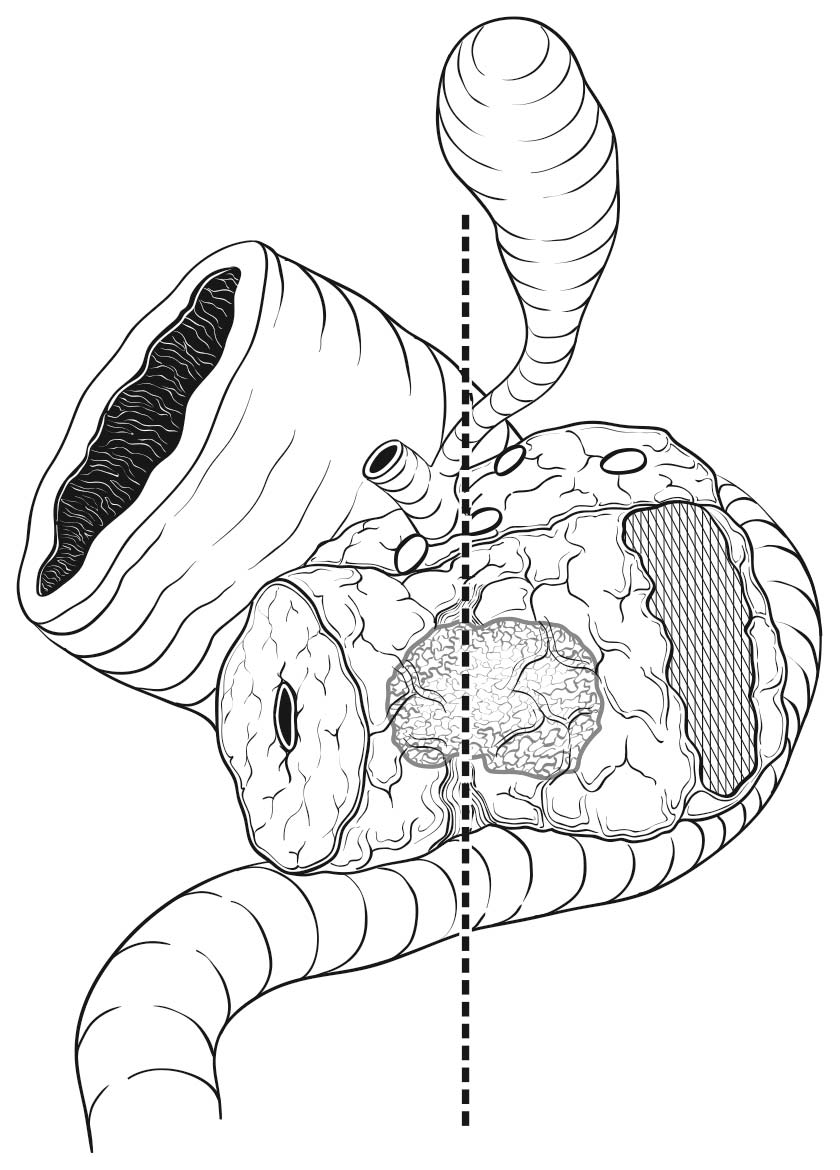
Most local recurrences arise in the pancreatic bed in the region of the uncinate margin. The soft tissue in this area is richly innervated and is adjacent to the right lateral aspect of the superior mesenteric artery (Figure 36.2). The uncinate margin should be inked as part of the gross evaluation of the specimen; the specimen is then cut perpendicular to the inked margin for histologic analysis. The closest microscopic approach of the tumor to the margin should be recorded. The smooth area adjacent to the uncinate process corresponds to the superior mesenteric and portal veins, and is referred to as the vascular groove or vascular bed. This area, as well as the posterior surface (including the nonuncinate posterior surface of the pancreatic head) and anterior surface (corresponding to the peritoneum), is not considered a true surgical margin in the CAP protocol, although this practice is not universally accepted. Histologic assessment of these areas for tumor is recommended but not mandated by the CAP protocol.
36.2 The retroperitoneal pancreatic margin (hatched area; also referred to as the mesenteric or uncinated margin) consists of soft tissue that often contains perineural tissue adjacent to the superior mesenteric artery.
The T categories are based on tumor size. For invasive carcinomas in association with intraductal mucinous neoplasm, intraductal tubulopapillary neoplasm, and mucinous cystic neoplasm, the T category should be determined by the size of the invasive component. The invasive carcinomas in this setting often are small and have a favorable outcome. These tumors have been referred to as minimally invasive carcinomas, with various criteria being used to define this term. T1 subcategories (T1a, T1b, and T1c) provide objective criteria for describing small invasive tumors, justifying the incorporation of these strata into the current system. The current cut-off points of less than or equal to 2 cm, greater than 2 to 4 cm, and greater than 4 cm for definitions of T1 to T3 are based on recent reviews of large databases.1,22-26 T4 for pancreatic cancer is defined as involvement of the superior mesenteric artery, celiac axis, and/or common hepatic artery, which in most cases renders the tumor unresectable. This situation usually is determined by radiographic and endoscopic findings. Hence, T4 category is not determined by pathological examination of surgical resection specimens. Adenocarcinomas of the head of the pancreas often show direct extension into the ampulla of Vater, intrapancreatic portion of the common bile duct, duodenum, peritoneum, and peripancreatic soft tissue. Adenocarcinomas of the body and tail of the pancreas may directly invade the stomach, spleen, left adrenal gland, and peritoneum. In the absence of arterial involvement (celiac axis, superior mesenteric artery, common hepatic artery), the T category is based on size, regardless of invasion of adjacent organs or veins. Extrapancreatic extension may be difficult to determine because the pancreas does not have a capsule, and the distinction between pancreas and extrapancreatic soft tissue often is obscured by fibrosis as part of the tumor or chronic pancreatitis. This parameter is no longer included in the definition of T categories.
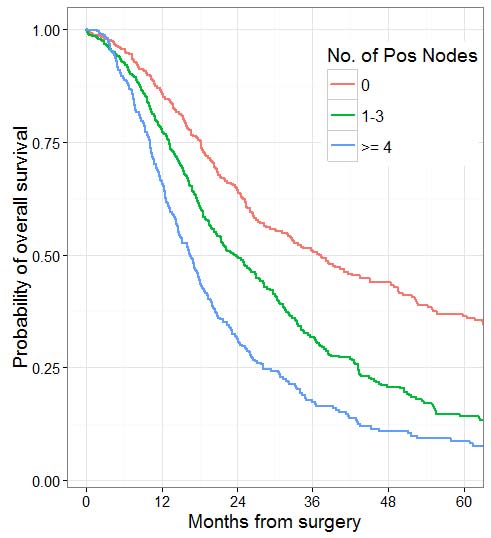
Nodal involvement, regardless of direct extension or metastases to peripancreatic nodes, has been associated with unfavorable outcomes in multiple studies.27 Thus, it is important to identify and properly assess as many regional lymph nodes in the specimen as possible. Based on survival data and a review of the number of lymph nodes that can be practically obtained from resection specimens, evaluation of a minimum of 12 lymph nodes is recommended to accurately stage N0 tumors.28,29 Recent studies show that the total number of positive lymph nodes and/or lymph node ratio (LNR) are also strong prognostic predictors.30-33 The total number of positive lymph nodes outperformed LNR in studies with sufficient numbers of lymph nodes obtained and evaluated.32,34 Thus, lymph node-positive categories based on the number of positive lymph nodes have been added to the N category classification of the pancreas, similar to other gastrointestinal sites. Although different cutoffs have been used in different studies,31,32,34 the cutoffs of zero versus one to three versus four or more have been adopted in the current staging scheme based on available data.34,35Anatomic division of regional lymph nodes is not necessary. However, separately submitted lymph nodes should be reported as labeled by the surgeon. Seeding of the peritoneum (even if limited to the lesser sac region), as well as peritoneal fluid with microscopic evidence of carcinoma, is considered M1.
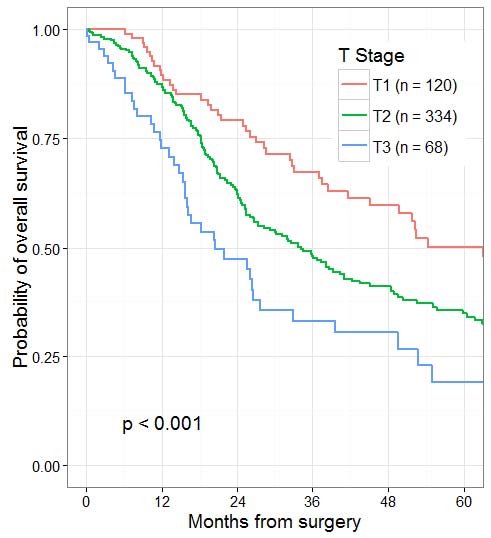
Patients who undergo surgical resection for localized nonmetastatic adenocarcinoma of the pancreas have a long-term survival rate of approximately 27% and a median survival of 12-20 months. Patients with resectable tumors showing regional lymph node involvement and without distant metastasis have a 5-year survival of approximately 11% and a median survival of 6-10 months. Patients with metastatic disease have a short survival (3-6 months), the length of which depends on the extent of disease, performance status, and response to systemic therapy.
- Preoperative CA 19-9
- Preoperative carcinoembryonic antigen (CEA)