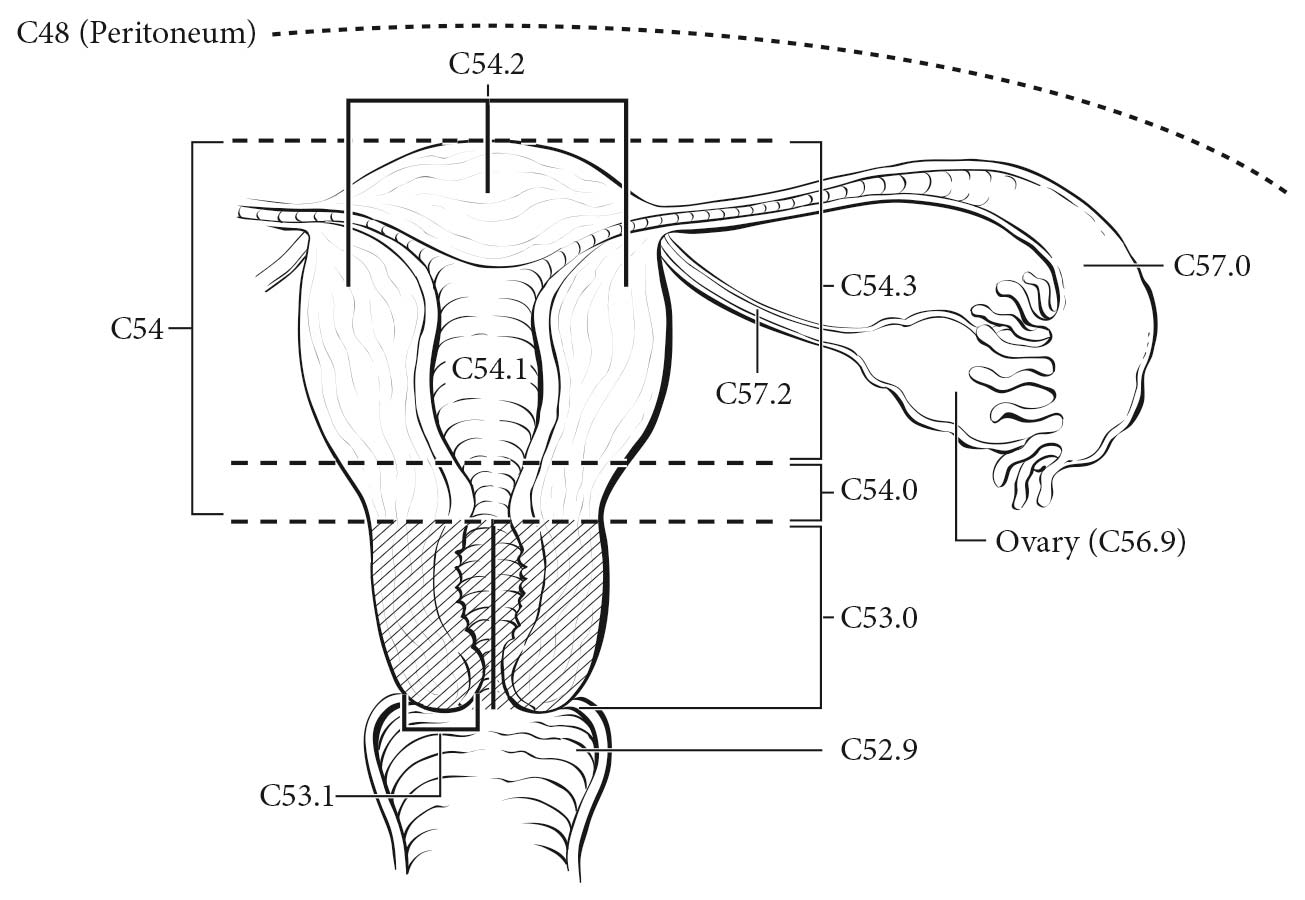
The ovaries are a pair of solid, flattened ovoids 2 to 4 cm in diameter that are connected by a peritoneal fold to the broad ligament and by the infundibulopelvic ligament to the lateral wall of the pelvis. They are attached medially to the uterus by the utero-ovarian ligament (Figure 68.1).
The fallopian tube extends from the posterior superior aspect of the uterine fundus laterally and anterior to the ovary. Its length is approximately 10 cm. The medial end arises in the cornual portion of the uterine cavity, and the lateral(fimbrial) end opens to the peritoneal cavity.
The peritoneum is the serous membrane of the abdominal cavity that lines the walls of the abdomen (parietal peritoneum) and covers the abdominal organs (visceral peritoneum). The pelvic peritoneum covers the fundus of the urinary bladder and the front of the rectum. In females, it lines the anterior and posterior surface of the uterus and the upper posterior vagina. There are two potential spaces posterior to the bladder (the uterovesical pouch) and posterior to the uterus (the rectouterine pouch of Douglas).On the anterior and posterior surfaces of the uterus, the peritoneum is reflected laterally to the pelvic sidewalls as the broad ligaments, containing the fallopian tubes.
Ovarian, fallopian tube, and peritoneal cancer is surgically and pathologically staged. There should be histologic confirmation of the ovarian, fallopian tube, and peritoneal disease. Laparotomy or operative laparoscopy with resection of the ovarian mass, as well as hysterectomy, forms the basis for staging. Biopsies of all frequently involved sites, such as the omentum, mesentery, diaphragm, peritoneal surfaces, pelvic nodes, and para-aortic nodes, are required for ideal staging of early disease. For example, to stage a patient confidently as Stage IA (T1 N0 M0), negative biopsies of all of the aforementioned sites should be obtained to exclude microscopic metastases. Assignment to Stage IIIA1 is based on spread to the retroperitoneal lymph nodes without intraperitoneal dissemination, because an analysis of these patients indicates that their survival is significantly better than that of patients with intraperitoneal dissemination.3,15-19 On the other hand, a single biopsy from an omental mass 2 cm or greater showing metastatic carcinoma is adequate to classify a patient as Stage IIIC, thus making other biopsies unnecessary from a staging standpoint. The final histologic and cytologic findings after surgery are to be considered in the staging. Operative findings before tumor debulking determine stage, which may be modified by histopathologic as well as clinical or radiologic evaluation (e.g., palpable supraclavicular node or pulmonary metastases on chest X-ray).
Although clinical assessments similar to those for other sites may be performed, surgical-pathological evaluation of the abdomen and pelvis is necessary to establish a definitive diagnosis of ovarian/fallopian tube/peritoneal cancer and to rule out other primary malignancies that may present with similar preoperative findings (e.g., bowel, uterine, and pancreatic cancers or, occasionally, lymphoma). Although laparotomy is the most widely accepted procedure for surgical-pathological staging, occasionally laparoscopy may be used. Occasionally, patients with advanced disease and/or women who are medically unsuitable candidates for surgery may be presumed to have ovarian cancer on the basis of cytology of ascites or pleural effusion showing typical carcinoma, combined with imaging studies demonstrating enlarged ovaries/fallopian tubes, and/or peritoneal involvement. Such patients usually are considered unstaged (TX), although positive cytology of a pleural effusion or supraclavicular lymph node occasionally allows designation of M1 or FIGO Stage IV disease. The presence of ascites does not affect staging unless malignant cells are present.
Imaging studies often are done in conjunction with definitive abdominal-pelvic surgery, and chest X-ray, bone scans, computed tomography (CT), or positron emission tomography (PET) may identify lung, bone, or brain metastases that should be considered in the final stage. Pleural effusions should be evaluated with cytology. In the future, pretreatment imaging will be more relevant to staging because of the increasing use of neoadjuvant chemotherapy in many women diagnosed with ovarian cancer.20
As with all gynecologic cancers, the final stage should be established at the time of initial treatment. It should not be modified or changed on the basis of subsequent findings.
Findings related to procedures such as laparoscopy or laparotomy after initial chemotherapy do not change the patient's original stage.
Imaging
CT currently is the preferred modality for the staging of ovarian cancer. Magnetic resonance (MR) imaging is excellent for characterizing adnexal masses. PET/CT is useful in evaluating distant disease but not for diagnosing primary ovarian cancer.
TNM Components of Tumor Staging
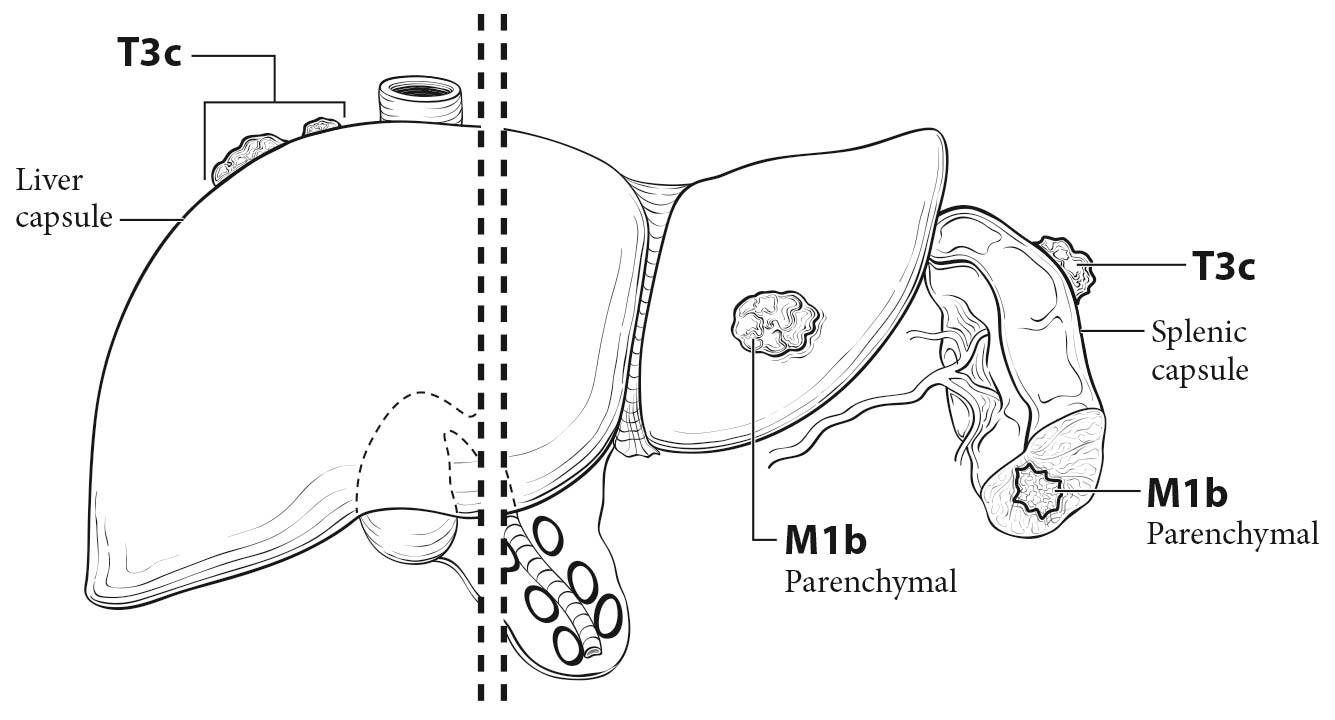
In clinical category T1, disease is limited to the ovaries; either MR imaging or ultrasound may be helpful in diagnosing the malignant adnexal mass. Contrast-enhanced CT is useful in assessing peritoneal disease. If the disease is confined to the pelvis, it is clinical category T2. Category T3a/b includes retroperitoneal lymph node metastases. PET/CT has been advocated to assess for lymph node metastases, as it has better specificity than contrast-enhanced CT or MR imaging. Category T3c includes surface involvement of the liver and spleen without any parenchymal metastases, which can be assessed with contrast-enhanced CT. PET/CT, if available, may be used as a single modality to assess both peritoneal disease and lung parenchymal disease in patients with advanced cancer.
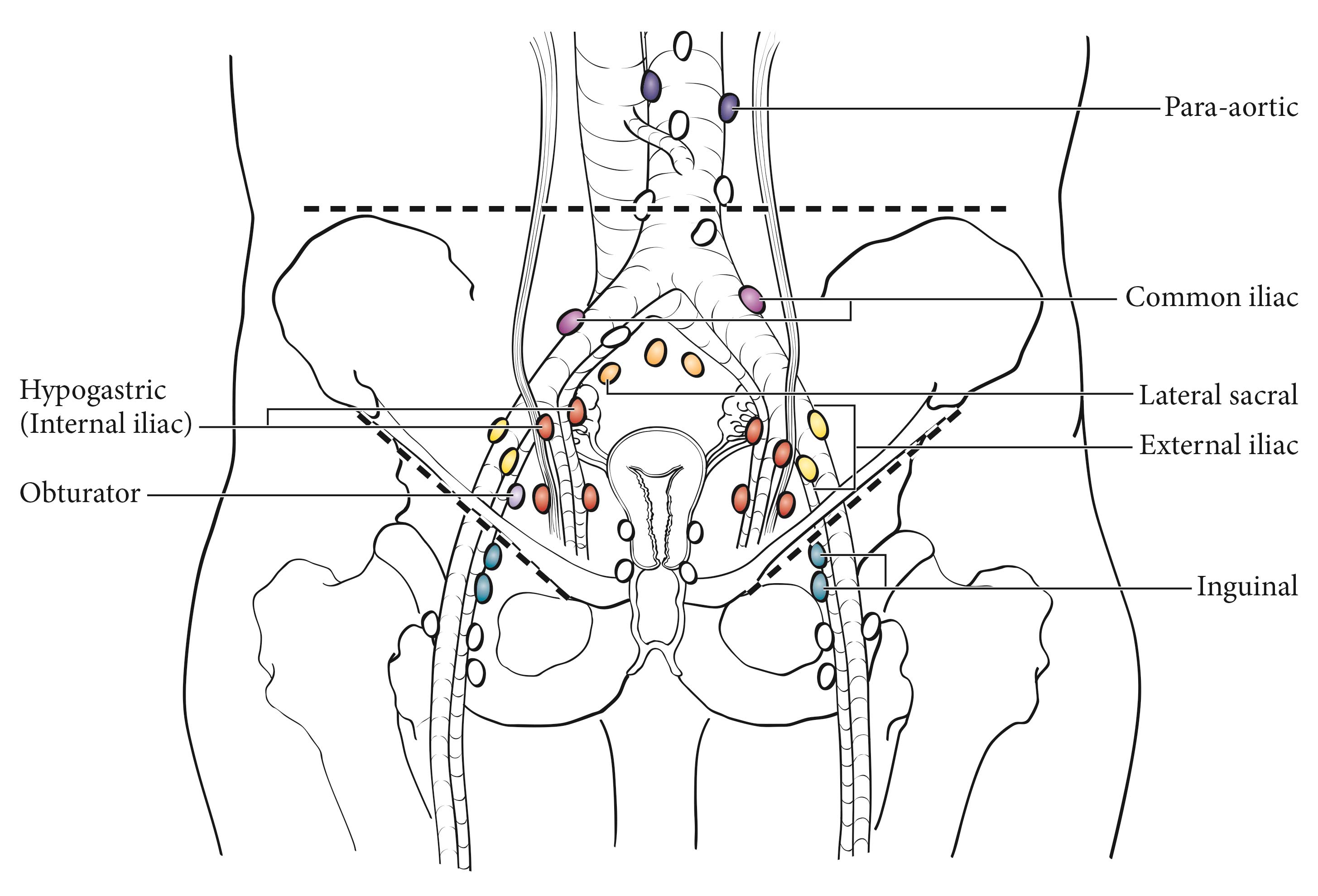
The imaging criteria used to assess lymph node metastases are based on node size, with abnormal being greater than 1 cm in the short axial dimension on cross-sectional scans. CT and MR imaging are shown to perform equally well in assessing adenopathy. However, because there may be false positive causes of enlarged nodes from benign disease, PET/CT is considered superior for assessing lymph node metastases. Metabolically active lymph nodes of any size on PET/CT are considered metastatic.
Suggested Imaging Report Format
- Primary tumor
- One or both ovaries
- Local extent
- Ascites
- Indicate whether localized to the pelvis or whether extrapelvic disease is present
- Retroperitoneal adenopathy
- Liver of splenic surface disease or lung parenchymal disease
- Pleural effusion
Surgery and biopsy of all suspected sites of involvement provide the basis for staging. Histologic and cytologic data are required. This is the preferred method of staging for ovarian cancer. The operative notes and/or the pathology report should describe the location and size of metastatic lesions and the primary tumors for optimal staging. In addition, the size of the tumor outside the pelvis must be determined, and noted and documented in the operative report. Size is reported in centimeters and represents the largest implant, regardless of whether it was resected during surgical exploration.
Carcinoma of the fallopian tube almost always is HGSC, which may be accompanied by STIC. The tumor invades locally into the muscular wall of the tube and then into the peritubal soft tissue or adjacent organs, such as the uterus or ovary, or through the serosa of the tube into the peritoneal cavity. Metastatic tumor implants may be found throughout the peritoneal cavity. The tumor may obstruct the tubal lumen and present as a ruptured or unruptured hydrosalpinx or hematosalpinx. It has been suggested that carcinomas in the fimbriated end without invasion have a worse prognosis than those invading the wall of the tube because of direct access to the peritoneal cavity.15
Examination of prophylactic salpingo-oophorectomy specimens from BRCA+ patients has shown thatmost early carcinomas detected in these samples occur in the tubal fimbria, and some of them arestill confined to the mucosa in the form of STIC.16,17 To detect these early carcinomas, serial longitudinal sections of the fallopian tube fimbria at 2- to 3-mm intervals should be obtained to examine most of the plicae surface.
Advanced invasive HGSC associated with STIC may be ovarian or tubal in origin without clinical relevance. For tumors limited to one ovary associated with STIC, there are three possibilities: a) STIC extending to one ovary, b) ovarian HGSC extending to the fallopian tube, and c) synchronous or metachronous tumor involving the ovary and fallopian tube. With regard to staging, these tumors are considered Stage IA ovarian carcinoma with STIC unless there is evidence of direct extension from STIC to the ovary, in which case they would be stage IIA fallopian tube carcinoma.
In some cases, an adenocarcinoma is primary in the peritoneum. The ovaries are not involved or are involved only with minimal surface implants. The clinical presentation, surgical therapy, chemotherapy, and prognosis of these peritoneal tumors mirror those of HGSC of the ovary. Patients who undergo prophylactic salpingo-oophorectomy for a familial history of ovarian cancer appear to retain a 1-2% chance of developing peritoneal adenocarcinoma, which is histopathologically and clinically similar to primary ovarian cancer. It is not possible to have Stage I peritoneal cancer.
Intranodal single tumor cells or small clusters of cells not more than 0.2 mm in greatest diameter are classified as isolated tumor cells. These may be detected by routine histology or by immunohistochemical methods. They are designated N0(i+).
For pN0, histologic examination should include both pelvic and para-aortic lymph nodes.
For patients receiving neoadjuvant therapy, it is important to record the clinical stage before treatment. Surgical staging after neoadjuvant therapy should be classified as “yp.”
- FIGO stage
- Preoperative CA-125 level
- Gross residual tumor after primary cytoreductive surgery
- Residual tumor volume after primary cytoreductive surgery
- Residual tumor location after primary cytoreductive surgery