Primary Site(s)
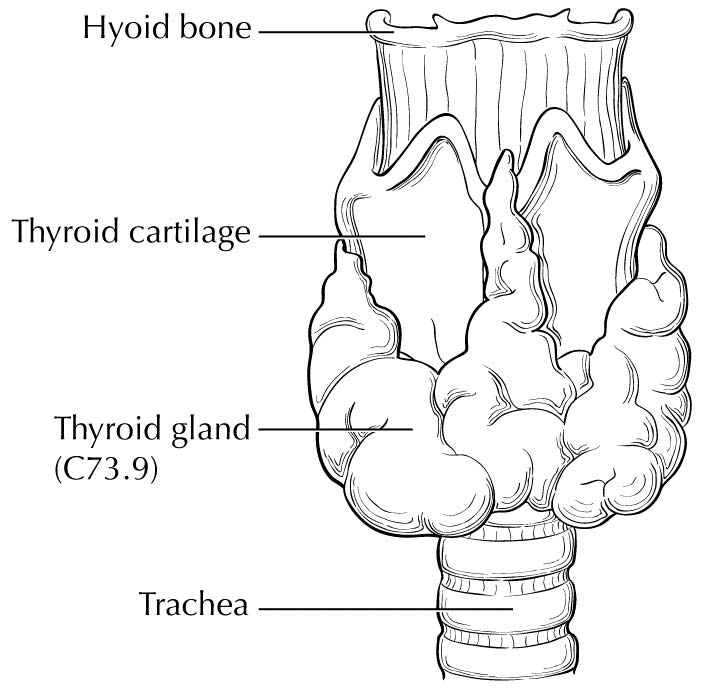
The thyroid gland ordinarily is composed of a right and a left lobe lying adjacent and lateral to the upper trachea and esophagus (Figure 93.1). An isthmus connects the two lobes, and in some cases, a pyramidal lobe is present, extending cephalad anterior to the thyroid cartilage.
Rarely, thyroid cancer may arise from thyroid follicular cells located outside the thyroid gland in locations such as the thyroglossal duct remnants, thyroid rests in the neck/upper mediastinum (thyrothymic tract), and ovaries (malignant struma ovarii).
Regional Lymph Nodes
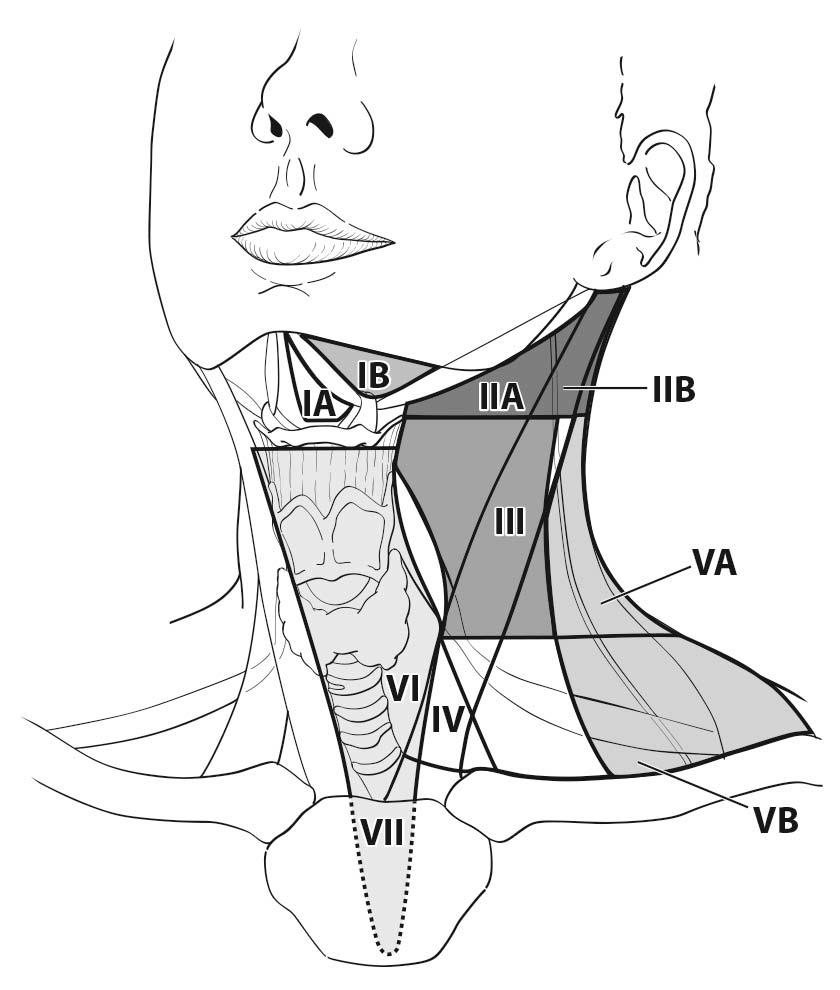
A seven-compartment nomenclature is commonly used to define anatomic lymph node compartment boundaries (Figure 93.2).24,25 The term central neck usually refers to levels VI and VII, whereas the lateral neck includes levels I, II, III, IV, and V. The first echelon of nodal metastasis most commonly includes the paralaryngeal, paratracheal, and prelaryngeal (Delphian) nodes adjacent to the thyroid gland.
Metastases also may involve the high (level IIA), mid- (level III), and lower jugular (level IV), and the supraclavicular (level V) and (less commonly) the upper deep jugular and spinal accessory lymph nodes (level IIB). Lymph node metastasis to submandibular and submental lymph nodes (level I) is rare. Upper mediastinal (level VII) nodal spread occurs frequently, both anteriorly and posteriorly. Retropharyngeal nodal metastasis may be seen, usually in the presence of extensive lateral cervical metastases. Bilateral nodal spread is common.
Clinical Classification
Most thyroid cancer patients present with asymptomatic nodules in the thyroid in the setting of normal thyroid function tests. Symptoms such as changes in the voice, dysphagia, or upper airway problems suggest more aggressive local disease. Distant metastases usually are identified as asymptomatic pulmonary nodules in high-risk patients but also may present as painful bone metastases or as masses causing local neurologic or vascular compromise.
Detailed guidance in preoperative assessments and management is provided in guidelines from the NCCN and the ATA.4,5 Preoperative staging for thyroid cancer typically includes neck ultrasound to evaluate the thyroid gland and central and lateral neck lymph node compartments. Fine-needle aspiration of suspicious thyroid nodules and/or abnormal-appearing lymph nodes should be undertaken preoperatively to obtain a definitive diagnosis and allow for appropriate surgical planning. FDG positron emission tomography (PET) scanning or neck computed tomography (CT)/magnetic resonance (MR) imaging is not recommended, except in patients in whom there is clinical suspicion of gross extrathyroidal extension or extensive, clinically apparent, cervical/mediastinal lymphadenopathy.
For staging purposes, the treatment date for most patients with differentiated thyroid cancer should be the date of thyroidectomy, because thyroid surgery is almost always the first step in treatment. Rarely, patients may receive external beam irradiation, chemotherapy, metastasectomy, or other neoadjuvant therapy as their initial treatment (before thyroid surgery or in patients who never undergo thyroid surgery). In these cases, the treatment date would correspond to the initiation of these other therapies, provided they begin before thyroid surgery.
The date of diagnosis should correspond to the first date of cytologic or histologic confirmation of thyroid cancer.
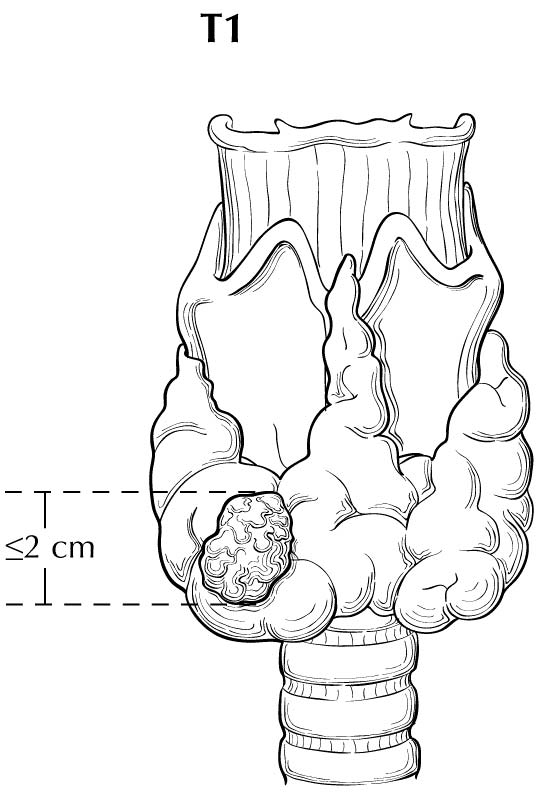
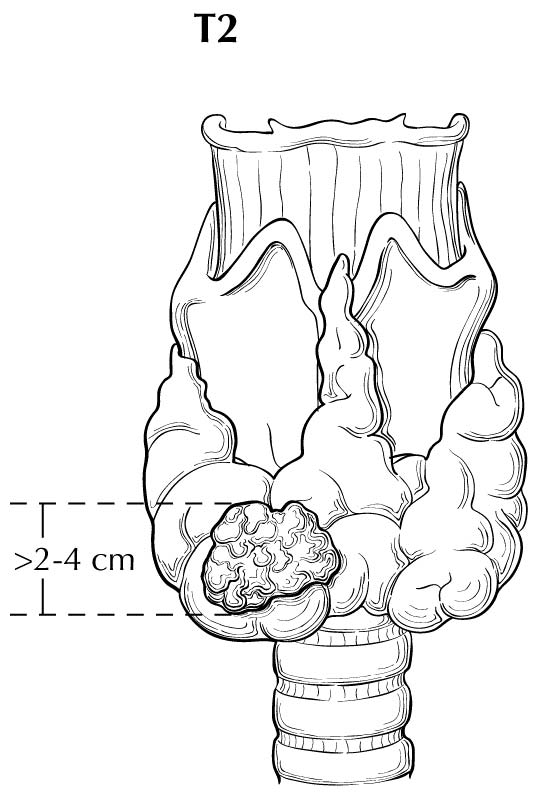
With regard to sizing of the primary tumor (T), this is almost always done based on the size of the largest differentiated thyroid cancer nodule within the thyroid gland, determined when the thyroid surgical specimen is processed for histologic examination. In situations in which the thyroid cancer is not surgically removed, the size of the primary lesion may be obtained by correlating cross-sectional imaging studies with biopsy results (by cytology or histology). These situations may become more common, as the 2015 ATA guidelines allow for an active surveillance management approach (observation instead of immediate surgery) in subcentimeter thyroid nodules that are either cytologically confirmed PTC or presumed to be thyroid cancer based on highly suspicious ultrasound characteristics.4 Although a highly suspicious ultrasound pattern carries a >70-90% likelihood that thyroid cancer is present,28-30 cytologic or histologic proof of disease is required before staging.
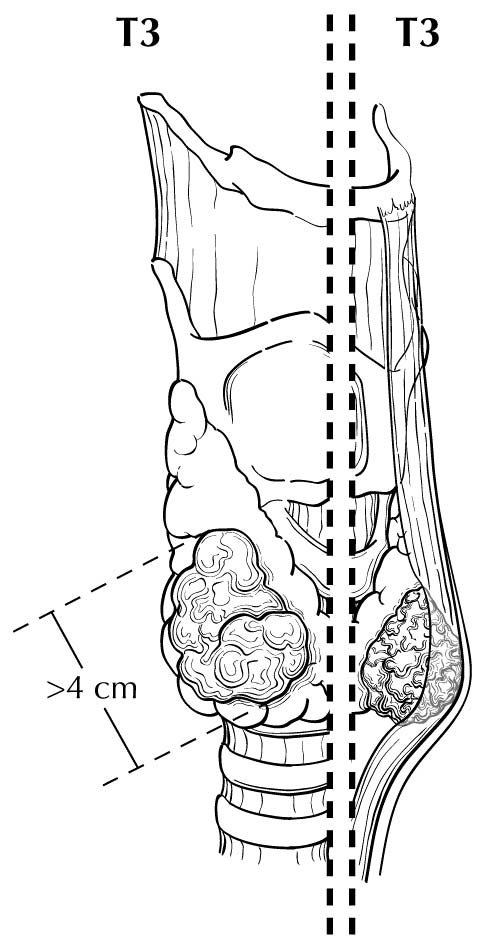
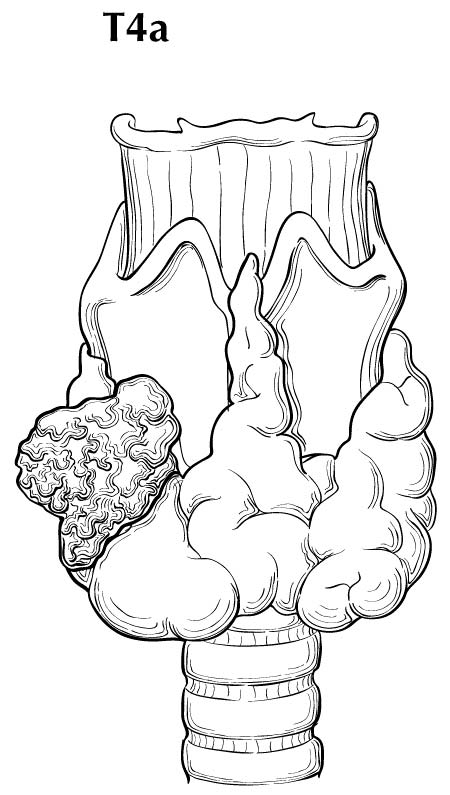
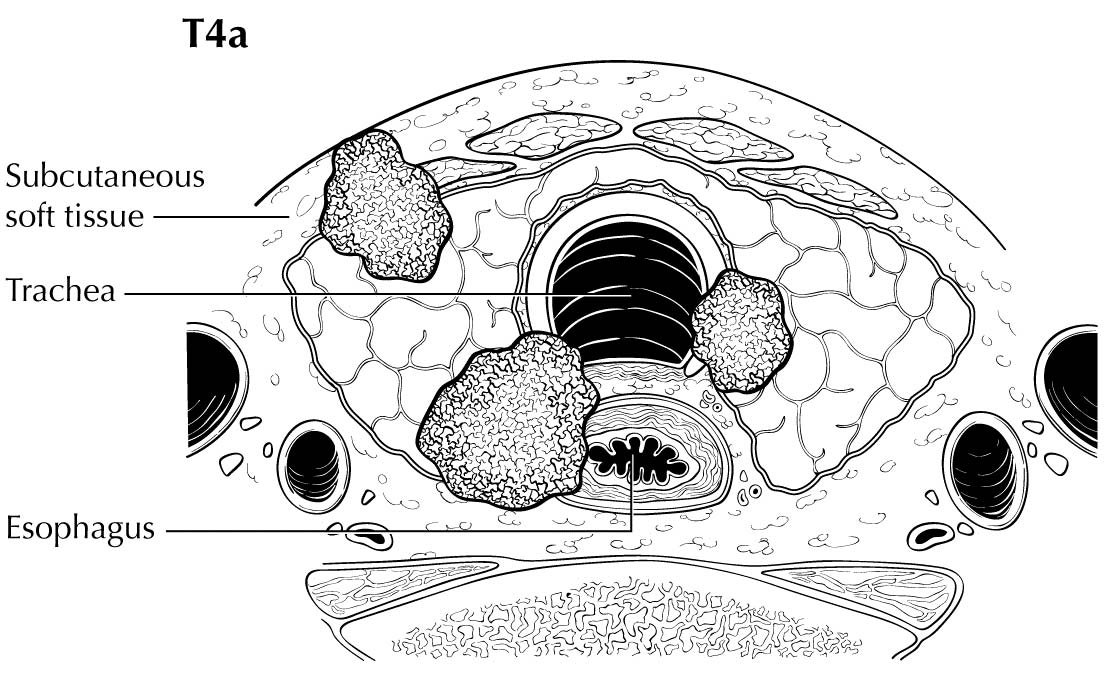
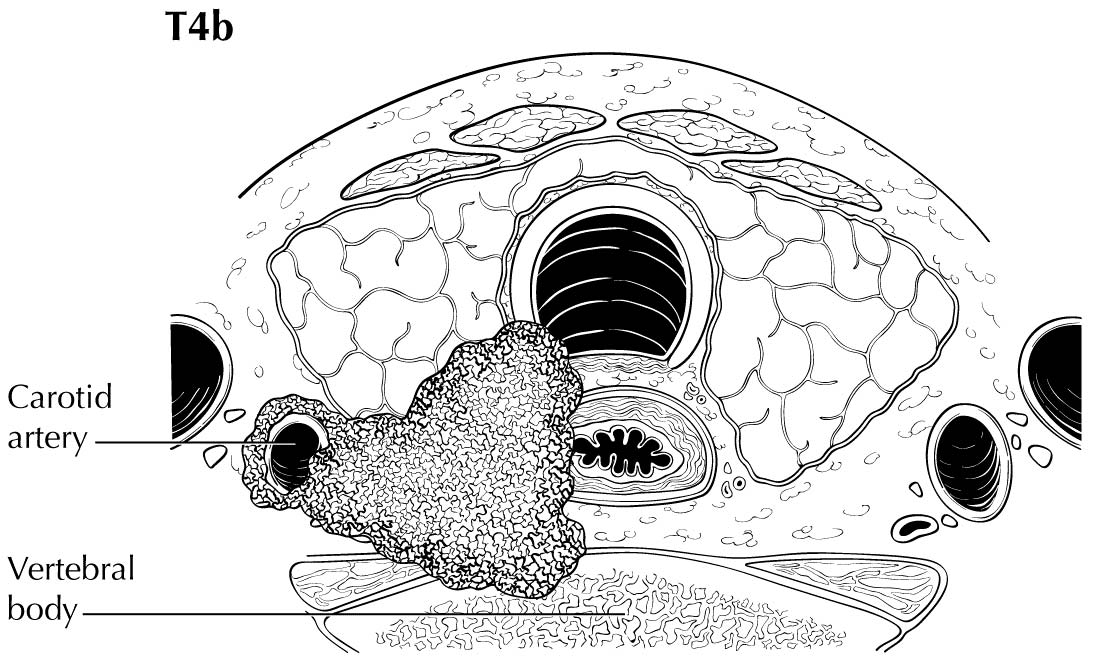
Extrathyroidal extension refers to the involvement of perithyroidal soft tissues by direct extension from the thyroid primary. Invasion outside the thyroid that can be identified clearly by imaging or intraoperative findings ranges from T3b disease (gross extrathyroidal extension involving only strap muscles) to T4a disease (demonstrating gross extrathyroidal extension invading the subcutaneous soft tissues, larynx, trachea, esophagus, muscle, or recurrent laryngeal nerve) to T4b disease (demonstrating gross extrathyroidal extension invading the prevertebral fascia or encasing the carotid artery or mediastinal vessels). The four infrahyoid muscles that either originate from or insert on the hyoid are often referred to as the strap muscles (including the sternohyoid, sternothyroid, thyrohyoid, and omohyoid muscles). Lesser degrees of extrathyroidal extension (minor) that are not clinically appreciated can be identified by microscopy as the tumor involves perithyroidal adipose tissue, strap muscles, nerves, or small vascular structures. Because the fibrous capsule of the thyroid is often incomplete, it is often difficult to determine whether the the boundary between thyroid cancer and fibroadipose tissue reflects an invasive process or simply the absence of a well-defined thyroid capsule in that area. For these reasons, and based on the lack of prognostic significance, the presence of minor extrathyroidal extension involving perithyroidal adipose tissue, strap muscles, nerves, or small vascular structures detected only by microscopy (not grossly evident) does not constitute T3b disease.
Clinical N1 disease (cN1) includes clinically apparent lymph node metastases (palpable or seen on imaging) that are either cytologically confirmed or highly suspicious for metastatic disease. Likewise, M1 status can be confirmed by cytologic/histologic assessment, documentation of RAI avidity of the metastatic lesion, or other imaging findings highly suspicious for distant metastasis in the proper clinical setting (e.g., inappropriately elevated serum thyroglobulin, ATA high-risk patient).
Imaging
Pretherapy Imaging
Preoperative neck ultrasonography to evaluate the thyroid gland as well as central and lateral neck lymph node chains is usually recommended as the initial staging procedure. Additional cross-sectional imaging with CT or MR imaging of the neck or distant sites is usually reserved for patients demonstrating clinical features of advanced disease, such as locally invasive primary tumor, clinically apparent multiple or bulky lymph node metastases, symptoms of distant metastases, or anaplastic thyroid cancers. Preoperative FDG-PET scanning is not routinely recommended but may be considered as part of initial staging in cases in which there is a reasonable likelihood of distant metastatic disease, such as in cases of poorly differentiated thyroid cancers, Hürthle cell cancers, and anaplastic thyroid cancers.4,5
Because of the high likelihood of both regional and distant metastases in anaplastic thyroid cancer, initial staging usually includes neck ultrasound; cross-sectional imaging of the head, neck, chest, abdomen, and pelvis with either CT or MR imaging; and/or FDG-PET scanning. At sites where PET scanning is performed using optimized PET/CT, the CT portion of the scan may supplant the need for additional anatomic imaging.4,5
These preoperative imaging examinations form the primary basis for preoperative clinical staging. Clinical T stage is based on the size of the primary tumor and an assessement of whether imaging demonstrates invasion of the tumor into the strap muscles, subcutaneous soft tissues, larynx, trachea, esophagus, recurrent laryngeal nerve, or prevertebral fascia or whether the tumor encases either the carotid artery or mediastinal vessels. The location of metastatic lymph nodes is used to define the clinical N stage (central neck vs. lateral neck disease). Most patients will be clinical M0, as routine use of cross-sectional or functional (RAI) imaging beyond the neck is not routinely performed, except in patients with locally advanced or anaplastic thyroid cancers.
One of the challenges with clinical staging is that nonspecific cervical lymphadenopathy is commonly found on routine ultrasonographic imaging and cannot be confidently classified as cN0 or cN1. In clinical practice, ultrasound-guided fine-needle aspiration of sonographically suspicious lymph nodes >=8 mm in smallest dimension is often performed if the results of the biopsy would alter initial management.4 Likewise, nonspecific pulmonary nodules also are quite common in the general population and usually cannot be confidently classified as benign or malignant findings before thyroid surgery.
Posttherapy ImagingMany patients undergo RAI scanning several weeks after thyroid surgery and at various time points during follow-up. These scans take advantage of the unique ability of most thyroid cells (both thyroid cancer and normal thyroid cells) to concentrate iodine. Although a focus of RAI uptake on the scan outside the thyroid bed usually indicates the presence of persistent or recurrent thyroid cancer, false positives do occur, which means that the RAI scans must be interpreted within the context of serum thyroglobulin and other patient risk factors for recurrence.
In most patients, neck ultrasonography is the primary imaging modality, with the testing interval based on initial risk stratification and the patient's response to therapy. Patients at high risk of regional or distant metastases also may be evaluated with cross-sectional imaging or FDG-PET scanning, depending on the serum thyroglobulin level and response to therapy classification.4
Because of the very high risk of recurrence and distant metastases, patients with anaplastic thyroid cancer require more frequent and extensive imaging. Cross-sectional imaging of the brain, neck, chest, abdomen, and pelvis is often performed at 1- to 3-month intervals for the first year of follow-up and then at 4- to 6-month intervals for an additional year. In addition, FDG-PET scanning is also considered at 3 to 6 months after initial therapy to identify persistent or recurrent disease.31
Radioiodine imaging is typically performed with either iodine-123 or iodine-131. Both are imaged with a conventional gamma camera, typically 24 to 48 hours after administration of the RAI. There is increasing interest in radioiodine imaging with an isotope of iodine that emits positrons, allowing the use of PET scanning for imaging. Iodine-124 has a half-life of 4.18 days, allowing for delayed imaging. PET scanning has a high sensitivity for detecting small-volume disease, and coacquisition with CT provides anatomic localization for therapy planning purposes. The quantitiative nature of PET scanning also allows for dosimetric therapy planning for radioiodine treatment using iodine-131, maximizing dose delivery to the tumor while limiting toxicity to bone marrow and other organs. This imaging technique has not yet achieved widespread acceptance, but it is being actively investigated at several sites.32
Pathological Classification
Pathological staging requires the use of all information obtained during clinical staging, as well as histologic study of the surgically resected specimen. The surgeon's description of gross extrathyroidal extension must also be included.
In this edition, the presence of minor extrathyroidal extension identified only on histologic examination and not apparent clinically is not used as a risk factor for staging. No distinction is made between tumors with and those without minor extrathyroidal extension. However gross extrathyroidal extension that can be identified clearly by imaging, intraoperative findings, or examination of tumor speciments is classified as T3b disease (gross extrathyroidal extension involving only strap muscles), T4a disease (gross extrathyroidal extension invading the subcutaneous soft tissues, larynx, trachea, esophagus, muscle, or recurrent laryngeal nerve), or T4b disease (gross extrathyroidal extension invading the prevertebral fascia or encasing the carotid artery or mediastinal vessels). Furthermore, because of the poorer survival outcomes associated with gross extrathyroidal extension, patients older than 55 years at diagnosis with T3b disease are classified as Stage II, those with T4a disease are classified as Stage III, and those with T4b disease are classified as Stage IV.
For staging purposes, “any N” includes pN0, pN1, pNX, cN0, or cN1 disease. Pathological confirmation of lymph node status is not required for staging purposes. Rather, patients with pNX disease who are cN0 are classified as “cN0/pNX” in the staging tables. As detailed in the section on the impact of regional lymph node metastasis on prognosis in differentiated thyroid cancer, subclinical (cN0) small-volume pN1 disease has little prognostic significance and is associated with outcomes that are very similar to those of pN0 disease. Because there is no requirement for a minimum number of lymph nodes to be sampled, pathological confirmation of one or more benign lymph nodes mandates a pN0 designation.
The identification of psammomatous calcifications within a cervical lymph node is considered metastatic disease and should be classified as N1 disease.
Complete assessment of N/M status may not be possible until after the RAI scans are complete, which often happens 1 to 3 months after initial surgery. Therefore, identification of metastatic disease (by any modality) within the first 4 months of thyroid surgery should be used to refine the N and M status.
Consistent with AJCC staging rules, the formal stage established during the first 4 months of follow-up does not change over time, even if the cancer progresses or recurs. However, the cancer may be “restaged” as new data become available during follow-up using the same approach and definitions applied during the initial staging. The lower case r is used to designate the restaging. In differentiated thyroid cancer, clinicians recognize both structural disease recurrence/progression (structural or functional evidence of disease) and biochemical disease recurrence/progression (abnormal thyroglobulin without structural or functional evidence of disease). Consistent with the approach to initial staging, restaging should be based only on the identification of structurally or functionally identifiable disease and not on the basis of abnormal biomarkers of disease (serum thyroglobulin or thyroglobulin antibodies).
- Histology
- Age at diagnosis
- Number of involved lymph nodes
- Maximum diameter of involved lymph nodes
- Size of largest metastatic foci within an involved lymph node