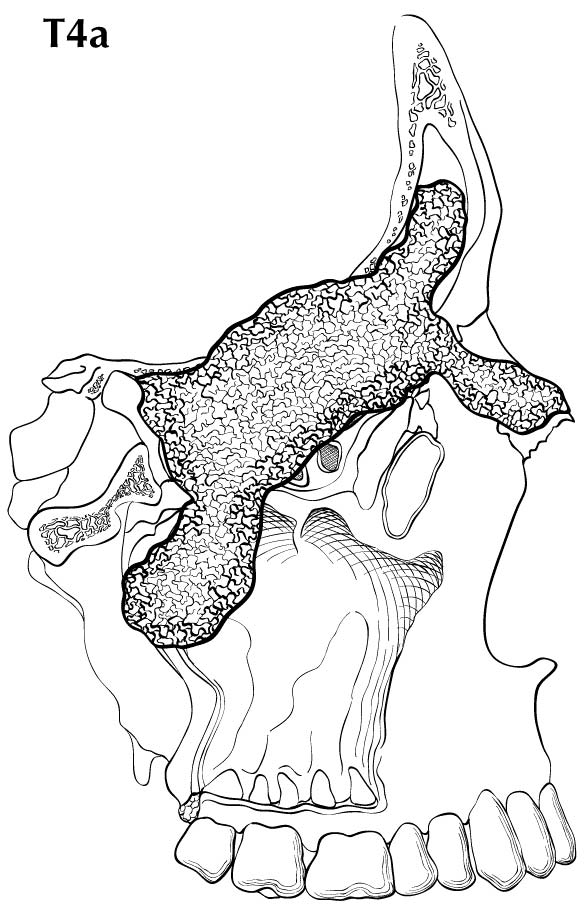
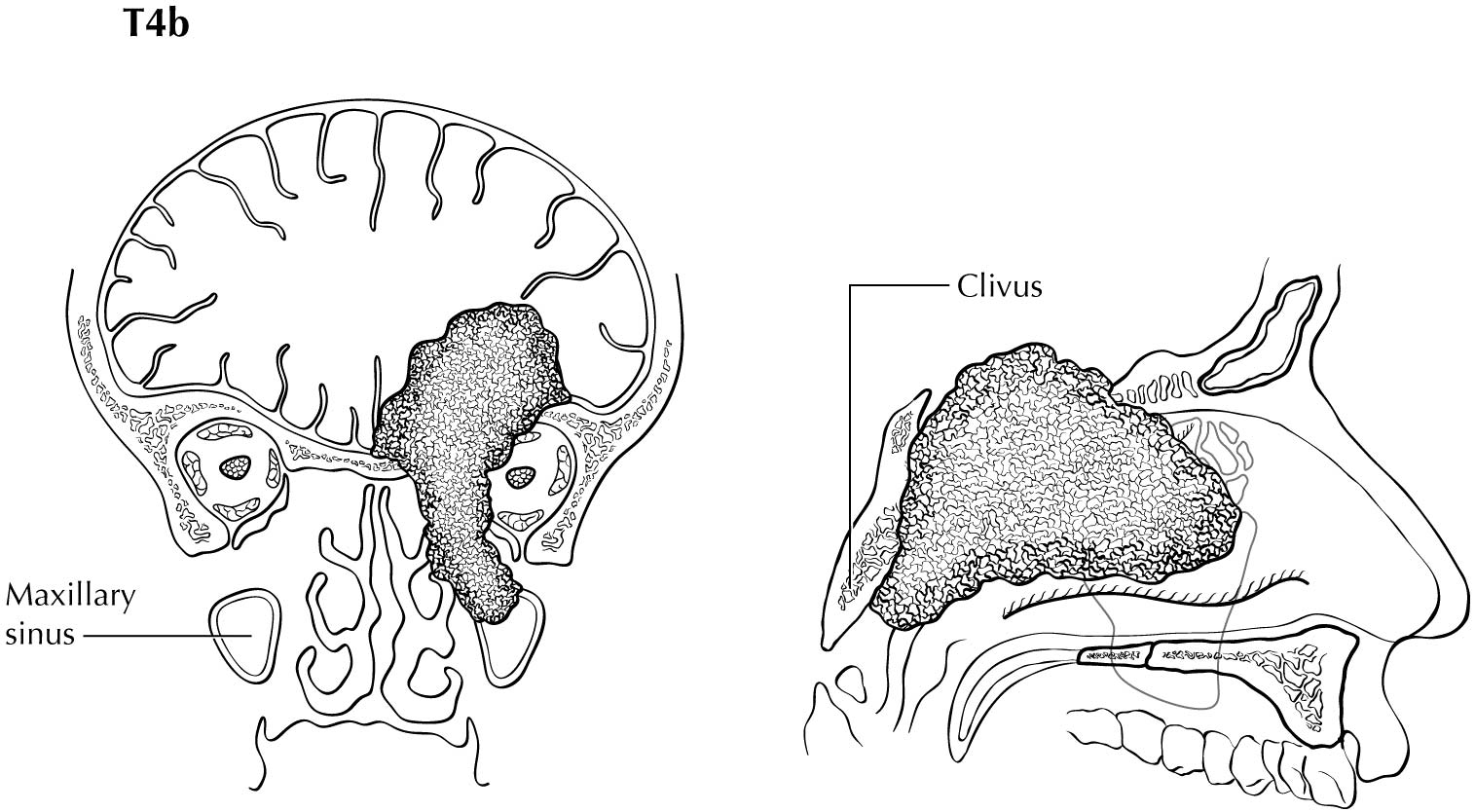
Primary Site(s)
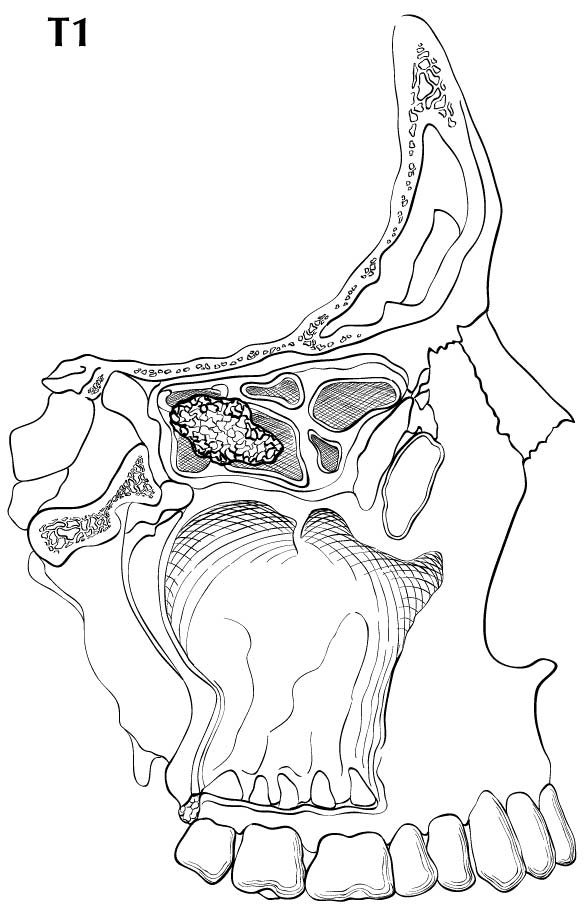
The location and the extent of the mucosal lesion within the maxillary sinus have prognostic significance. Historically, a plane connecting the medial canthus of the eye to the angle of the mandible, represented by Ohngren's line, is used to divide the maxillary sinus into an anteroinferior portion (infrastructure), which is associated with a good prognosis, and a posterosuperior portion (suprastructure), which has a poor prognosis (Figure 14.1). The poorer outcome associated with suprastructure cancers reflects early invasion by these tumors to critical structures, including the orbit, skull base, pterygoid plates, and infratemporal fossa.
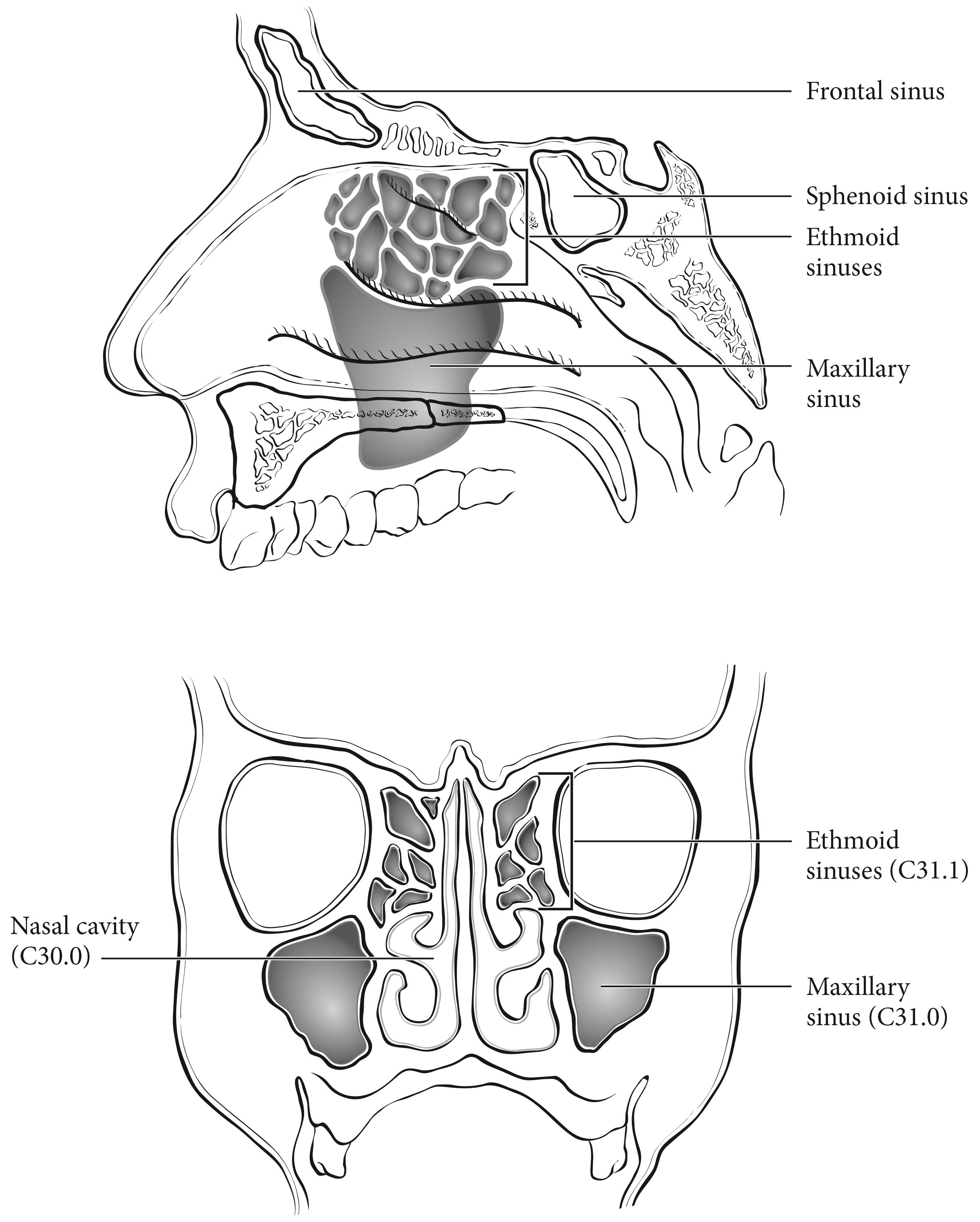
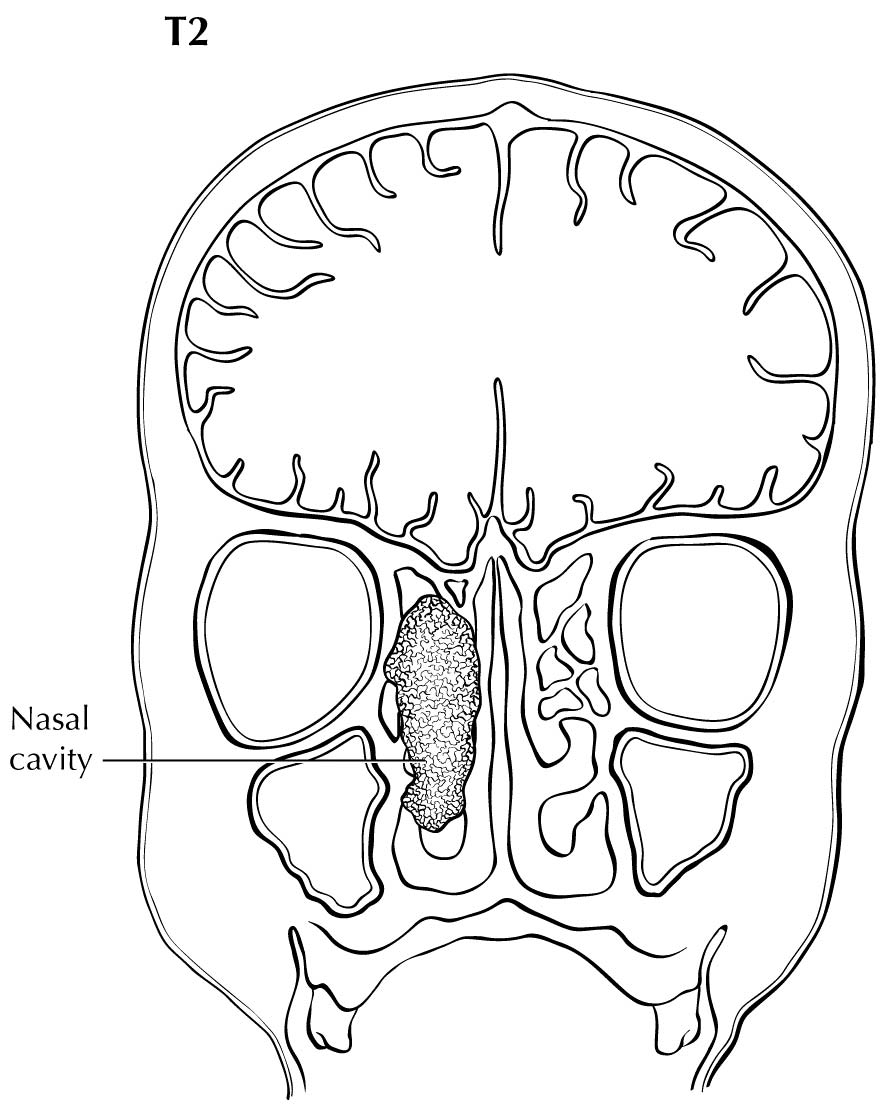
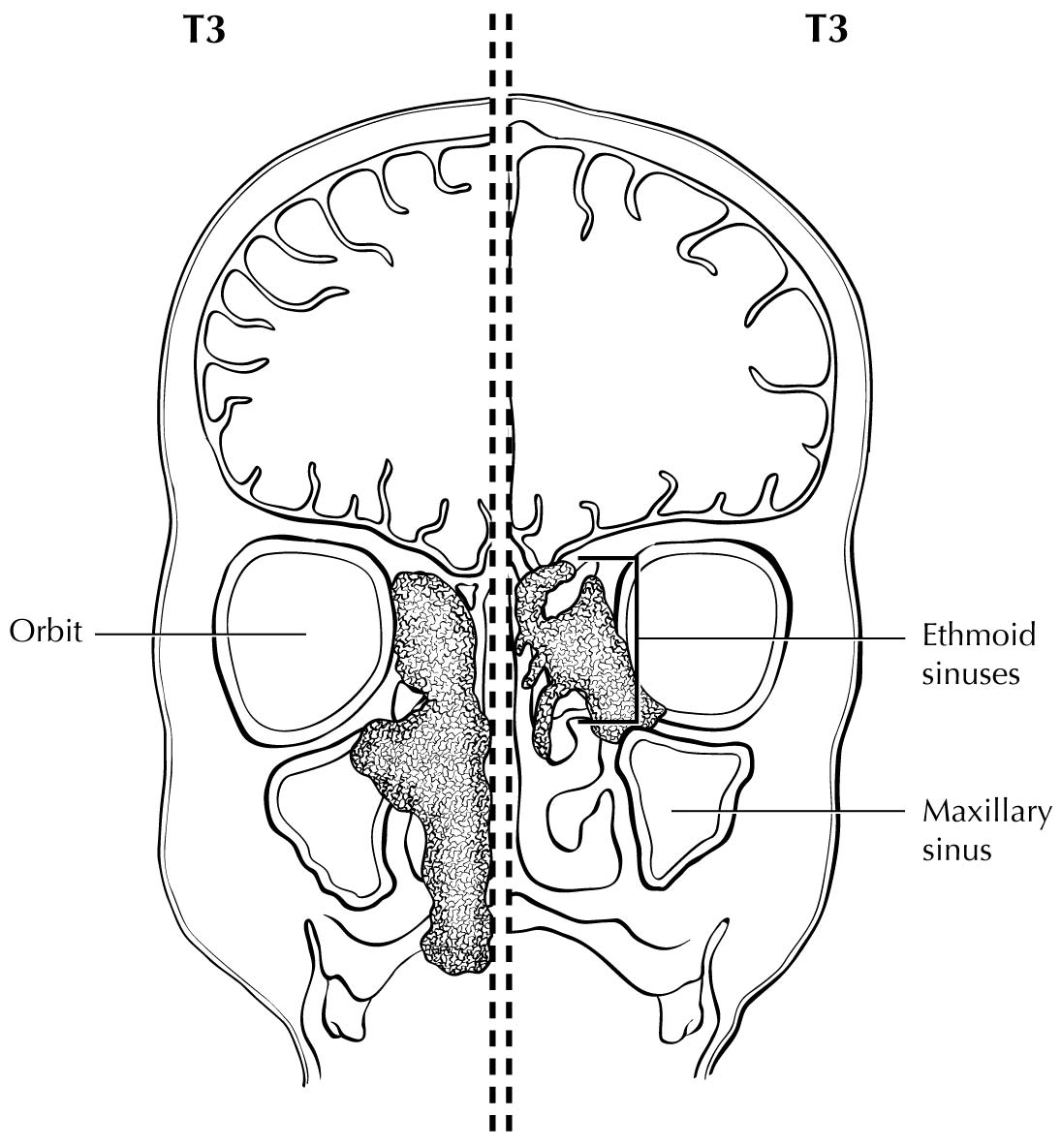
For the purpose of staging, the nasoethmoidal complex is divided into two sites: nasal cavity and ethmoid sinuses. The ethmoids are further subdivided into two subsites: left and right, separated by the nasal septum (perpendicular plate of ethmoid). The nasal cavity is divided into four subsites: the septum, floor, lateral wall, and the edge of naris to mucocutaneous junction.
| Site | Subsite(s) |
|---|---|
| Maxillary sinus | Left/right |
| Nasal cavity | Septum |
| Floor | |
| Lateral wall | |
| Edge of naris to mucocutaneous junction | |
| Ethmoid sinus | Left/right |
Regional lymph node spread from cancer of nasal cavity and paranasal sinuses is relatively uncommon. Involvement of buccinator, prevascular facial, submandibular, upper jugular, and (occasionally) retropharyngeal nodes may occur with advanced maxillary sinus cancer, particularly those extending beyond the sinus walls to involve adjacent structures, including soft tissues of the cheek, upper alveolus, palate, and buccal mucosa or overlying skin. Ethmoid sinus cancers are less prone to regional lymphatic spread. When only one side of the neck is involved, it should be considered ipsilateral. Bilateral spread may occur with advanced primary cancer, particularly with spread of the primary beyond the midline.
Clinical Classification
The assessment of primary maxillary sinus, nasal cavity, and ethmoid tumors is based on inspection and palpation, including examination of the orbits, nasal and oral cavities, and nasopharynx, and neurologic evaluation of the cranial nerves. Nasal endoscopy with rigid or fiberoptic flexible instruments is recommended for inspection and biopsy.
Neck nodes are assessed by palpation. In clinical evaluation, the maximum size of any nodal mass should be measured. Biopsy, when indicated, is done with a fine needle and not open approach and is included in clinical classification if done. The three categories of clinically involved nodes for the nasal cavity and paranasal sinus are N1, N2, and N3. Midline nodes are considered ipsilateral nodes. Superior mediastinal lymph nodes are considered regional lymph nodes (level VII). In addition to the components to describe the N category, regional lymph nodes should also be described according to the level of the neck that is involved. Provide a description or map of the regional lymph nodes and node groups that the cancer affects. Unambiguous evidence of gross ENE (i.e., defined as invasion of skin, infiltration of musculature/fixation to adjacent structures on clinical examination, cranial nerve, brachial plexus, sympathetic trunk or phrenic nerve invasion with dysfunction) is a sufficiently high threshold to classify these as clinical ENE(+). Examinations for distant metastases include appropriate imaging, blood chemistries, blood count, and other routine studies as indicated. Biopsy is typically needed to confirm metastasis, although a risk-to-benefit ratio is always weighed and is included in clinical stage as pM1 if done.
Imaging studies showing amorphous spiculated margins of involved nodes or involvement of internodal fat resulting in loss of normal oval-to-round nodal shape strongly suggest extracapsular (extranodal) tumor spread. No imaging study (as yet) can identify microscopic foci in regional nodes or distinguish between small reactive nodes and small malignant nodes without central radiographic inhomogeneity.
Imaging
Imaging is beneficial for lesions that cannot be fully assessed on clinical examination, locally advanced disease, or symptomatic patients. Computed tomography (CT) and magnetic resonance (MR) imaging are complementary imaging studies for staging patients with cancers involving the nasal cavity and/or the paranasal sinuses. There is no indication for plain films or positron emission tomography (PET)-CT for staging of the primary site. PET-CT may be helpful for assessing nodal metastases; however, lymph node staging is discussed in Chapter 6.
CT is superior to MR imaging for identifying bone erosion of thin walls and septa of the paranasal sinuses. CT is superior to MR imaging for identifying involvement of the hard palate. Either CT or MR imaging may be performed for tumor extending outside of the nose and/or paranasal sinuses to involve the adjacent structures, including the orbital apex (T4b). MR imaging, especially with T2-weighted images, is helpful for tumor mapping and for distinguishing between tumor extension and obstructed secretions. Tumors may obstruct the frontal recess or sphenoethmoidal recess, which may result in obstruction of the frontal or sphenoid sinus, respectively. Differential between tumor extension and proteinaceous obstructed secretions is best performed with MR imaging, which will help accurately identify T4a tumors. Both CT and MR imaging may be used to evaluate for posterior spread to the pterygopalantine fossa. However, MR imaging is superior to CT for evaluating for retrograde perineural spread along V2 through foramen rotundum or V3. CT is superior to MR imaging for early cortical involvement, but MR imaging is superior to CT for bone marrow invasion. MR imaging also is superior to CT for identifying dural involvement or other types of intracranial extension (T4b).
Pathological Classification
Complete resection of the primary site and/or regional nodal dissections, followed by pathological examination of the resected specimen(s), allows the use of this designation for pT and/or pN, respectively. Specimens that are resected after radiation or chemotherapy need to be identified and considered in context, and use yp instead of p. pT is derived from the invasion of bone, orbit, dura, and presence of disease in multiple subsites. Pathological staging represents additional and important information and should be included as such in staging, but it does not supplant clinical staging as the primary staging scheme.
For pN, a selective neck dissection will ordinarily include 10 or more lymph nodes, and a radical or modified radical neck dissection will ordinarily include 15 or more lymph nodes. Negative pathological examination of a smaller number of nodes still mandates a pN0 designation.
Definition of ENE and Description of Its Extent
All surgically resected metastatic nodes should be examined for the presence and extent of ENE. The precise definition of ENE has varied in the literature over the course of time. The American College of Pathologists defines ENE as extension of metastatic tumor, present within the confines of the lymph node, through the lymph node capsule into the surrounding connective tissue, with or without associated stromal reaction.
ENE detected on histopathologic examination is designated as ENEmi (microscopic ENE less than or equal to 2 mm) or ENEma (major ENE greater than 2 mm). Both ENEmi and ENEma qualify as ENE(+) for definition of pN. These descriptors of ENE will not be required for current pN definition, but data collection is recommended to allow standardization of data collection and future analysis.
- ENE clinical status: ENE(-) or ENE(+)
- ENE pathological status: ENE(-) or ENE(+)
- The extent of microscopic ENE (distance of extension from the native lymph node capsule to the farthest point of invasion in the extranodal tissue)
- Perineural invasion
- Lymphovascular invasion
- Performance status
- Tobacco use
- Alcohol use
- Depression diagnosis