- Pharmacology. Ethanol (ethyl alcohol) acts as a competitive substrate for the enzyme alcohol dehydrogenase, preventing the formation of toxic metabolites from methanol or ethylene glycol. A serum ethanol concentration of 100 mg/dL, or at least a 1:4 molar ratio of ethanol to toxic alcohol/glycol, effectively saturates alcohol dehydrogenase and prevents further methanol and ethylene glycol metabolism (see also “Fomepizole [4-Methylpyrazole, 4-MP],”). Ethanol is well absorbed from the GI tract when given orally, but the onset is more rapid and predictable when it is given intravenously. The elimination of ethanol is zero order; the average rate of decline is 15 mg/dL/h. However, this is highly variable and will be influenced by prior chronic use of alcohol, recruitment of alternate metabolic pathways, and concomitant hemodialysis (eg, to remove methanol or ethylene glycol).
- Indications. Suspected methanol (methyl alcohol) or ethylene glycol poisoning with the following:
- A history suggestive of ingestion but no available blood concentration measurements;
- An elevated anion gap metabolic acidosis unexplained by ketones, lactate or uremia.
- An unexplained elevated osmol gap; or
- A serum methanol or ethylene glycol concentration of 20 mg/dL or higher.
- Note: Since the introduction of fomepizole (4-methylpyrazole), a potent inhibitor of alcohol dehydrogenase, most patients with ethylene glycol or methanol poisoning probably will be treated with this drug instead of ethanol, particularly in cases involving small children, patients taking disulfiram, patients with pancreatitis, and hospitals lacking laboratory support to perform rapid ethanol levels (for monitoring treatment). Ethanol is more difficult to dose, requires more monitoring, and has a greater risk of adverse effects. Studies suggest that despite the higher acquisition costs for fomepizole, it may be more cost-effective than ethanol.
- Other substances that are metabolized by alcohol dehydrogenase to toxic metabolites include propylene glycol, diethylene glycol, triethylene glycol, glycol ethers (eg, ethylene glycol ethyl ether, ethylene glycol butyl ether), and 1,4-butanediol. The criteria for ethanol therapy and evidence for improved outcomes are lacking for these substances.
- Contraindications. Use of interacting drugs, which may cause disulfiram-type reaction (see Item V. B below).
- Adverse effects
- Nausea, vomiting, and gastritis may occur with oral administration. Ethanol may also exacerbate pancreatitis.
- Inebriation, sedation, and hypoglycemia (particularly in children and malnourished adults).
- Intravenous use is sometimes associated with local phlebitis (especially with ethanol solutions >10%). Hyponatremia may result from large doses of sodium-free intravenous solutions.
- Acute flushing, palpitations, and postural hypotension may occur in patients with atypical aldehyde dehydrogenase enzyme (up to 35-40% of those with East Asian descent and up to 8% of the global population).
- Use in pregnancy. FDA Category C (indeterminate). Ethanol crosses the placenta. Chronic use in pregnancy is associated with birth defects (fetal alcohol syndrome). The drug reduces uterine contractions and may slow or stop labor. However, these effects do not preclude its acute, short-term use for a seriously symptomatic patient (Introduction).
- Drug or laboratory interactions
- Ethanol potentiates the effect of CNS-depressant drugs and hypoglycemic agents.
- Disulfiram reaction, including flushing, palpitations, and postural hypotension, may occur in patients taking disulfiram as well as a variety of other medications (eg, metronidazole, furazolidone, procarbazine, chlorpropamide, some cephalosporins, and Coprinus mushrooms). In such cases, fomepizole is the recommended alternative to ethanol treatment.
- Drugs or chemicals metabolized by alcohol dehydrogenase (eg, chloral hydrate, isopropyl alcohol) also have impaired elimination. Fomepizole inhibits the metabolism of ethanol, and vice versa.
- Dosage and method of administration. See Table III-10. Ethanol may be given orally or intravenously. The desired serum concentration is approximately 100 mg/dL (20 mmol/L). Note: Ethanol formulations in percent (%) are normally expressed as volume of ethanol per volume of solution (mL/dL) instead of weight per volume (g/dL). The specific gravity of ethanol (0.79 g/mL) is less than that of water (1 g/mL); to convert mL/dL to g/dL, multiply by 0.79. A 10% ethanol solution is preferred for IV administration (to minimize fluid load, but it may require central venous access in children); a solution of 20% (usually diluted with juice for better palatability and absorption) is preferred for oral administration.
- Loading dose. Give approximately 800 mg/kg as a loading dose, unless the patient already has an elevated ethanol level, in which case the loading dose should be reduced by a proportional amount (see Table III-10 footnote).
- Maintenance dose. Administer approximately 100-150 mg/kg/h (give the larger dose to persons with chronic alcoholism). Obtain serum ethanol levels after the loading dose and frequently during maintenance therapy to ensure a concentration of 100 mg/dL (eg, every 1-2 hours until goal achieved or after a change in the infusion rate, then every 2-4 hours during the maintenance dosing).
- Dosing during hemodialysis. Increase the maintenance infusion rate to 175-350 mg/kg/h (use the larger dose for persons with chronic alcoholism) during hemodialysis to offset the increased rate of ethanol elimination.
| Dose | Intravenousa | Oralb 20% (40 Proof) | |
|---|---|---|---|
| 5% | 10% | ||
| Loadingc | 20 mL/kg | 10 mL/kg | 5 mL/kg |
| Maintenanced | 2.5-4 mL/kg/h | 1.25-2 mL/kg/h | 0.5-1 mL/kg/h |
| Maintenance during hemodialysisd | 4.5-8 mL/kg/h | 2.25-4 mL/kg/h | 1-1.7 mL/kg/h |
a% is mL ethanol/100 mL (mL/dL). Infuse intravenous loading dose over 20-60 minutes as tolerated. For slower rates, add 1 mL/kg to the loading dose to account for ethanol metabolism during the infusion.
b% is mL ethanol/100 mL (mL/dL). Dilute to an ethanol concentration of 20% or less and administer orally or by nasogastric tube.
cIf the patient's serum ethanol level is greater than zero, reduce the loading dose in a proportional manner. Multiply the calculated loading dose by the following factor: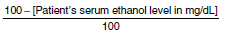
dDoses may vary according to the individual. Persons with chronic alcoholism have a higher rate of ethanol elimination, and maintenance doses should be adjusted to maintain an ethanol level of approximately 100-150 mg/dL.