- The clinical utility of Phenobarbital levels include:
- To monitor compliance with therapeutic regimen in the treatment of epilepsy, prevention of seizures, and sometimes as a mood stabilizer
- To monitor and evaluate change of medication or dosage
- Evaluation of overdose and prevention of toxicity, especially in combination with ethanol
- To monitor in children, elderly people, and persons with poorly controlled seizures or if patient noncompliance is suspected
- To time reinstitution of chronic therapy after overdose
- Phenobarbital toxicity may clinically present as:
- Cardio-Respiratory
- Apnea (Respiratory arrest)
- Bradycardia
- Bradypnea
- Cyanosis
- Hypotension
- Oliguria
- Shock
- Slow/Periodic breathing (Cheyne-Stokes)
- CNS
- Areflexia/Hyporeflexia
- Ataxia
- Coma
- Delirium
- Depression
- Hyperkinesis (Paradoxical)
- Hypothermia
- Irritability
- Slurred speech
- Somnolescence
- Integumentary
- Blisters and bullae at the sites of friction and pressure (hands, knees, buttocks, etc.)
- Cold, clammy skin
- Ocular
- Diplopia
- Nystagmus
- Alternating constriction and dilatation of pupil
- Others
- Coarsening of facial features
- Decreased libido
- Dupuytren contractures
- Megaloblastic anemia
- Osteomalacia
- Rapid absorption has made phenobarbital a common choice for treatment of seizures in infants, but cognitive and behavior alterations have led to decreased use in older children and adults
- Children are more likely than adults to exhibit behavioral changes (paradoxical hyperkinesis)
- Drug interactions of phenobarbital include:
- Increases the effectiveness of drugs such as:
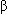 -Blockers
-Blockers- Corticosteroids
- Haloperidol
- Methylphenidate
- Phenothiazines
- Phenylbutazone
- Phenytoin
- Propoxyphene
- Quinidine
- Tricyclic antidepressants
- Valproic acid
- Decreases the effectiveness of drugs such as:
- Chloramphenicol
- Cyclosporine
- Doxycycline
- Ethosuximide
- Griseofulvin
- Oral anticoagulants
- Oral contraceptives
- Phenytoin
- Theophylline
- Phenobarbital co-administered with sedative drugs or ethanol has an additive effect
Additional information
- Phenobarbital after oral ingestion reaches the peak plasma level in 1-3 hours and in minutes following an IV infusion
- Phenobarbital has plasma protein binding of 40-60% and bioavailability of 80-100% in adults
- Phenobarbital is primarily metabolized by the liver with a major metabolite that is p-hydroxyphenobarbital, which is excreted in the urine as a glucuronide conjugate
- The plasma half-life in infants is up to 400 hours, 20-75 hours in children >6 months of age, and 75-120 hours in adults
- After IV administration, Phenobarbital is distributed quickly to highly vascular organs, except the brain, and then it is distributed evenly. After 6-12 minutes, it penetrates the brain; however, penetration into the brain is more rapid during status epilepticus due to increased blood flow and acidosis
- Many factors must be considered in effective dosing and monitoring of therapeutic drugs including patient age, weight, drug, route of administration, interacting medications, electrolyte balance, protein levels, water balance, conditions that affect absorption and excretion, and ingestion of other substances (foods, herbals, vitamins, and minerals) that can either potentiate or inhibit the intended target concentration
- Factors interfering with test results include:
- Sample collection in serum separator tubes/gel tubes (gel slowly absorbs the drug)
- Serum frozen on cells or left standing on cells for several days
- Hemolysis, lipemic, or icteric specimen
- Hepatic or renal disease
- Related laboratory tests include:
- Albumin
- Blood urea nitrogen (BUN)
- Calcium
- Cerebrospinal fluid (CSF) examination
- Complete blood count (CBC)
- Creatinine
- Electrolytes
- Ethanol level
- Glucose
- Illicit drug screen (blood or urine)
- Liver function tests
- Magnesium
- Thyroid stimulating hormone (TSH)
- Total protein
This section covers Antiseizure medication levels - Phenobarbital. Other sections provide detailed information on other components of Antiseizure medication levels.
- Antiseizure medication levels - Overview
- Antiseizure medication levels - Benzodiazepines
- Antiseizure medication levels - Carbamazepine
- Antiseizure medication levels - Ethosuximide
- Antiseizure medication levels - Other agents
- Antiseizure medication levels - Phenytoin
- Antiseizure medication levels - Primidone
- Antiseizure medication levels - Valproic acid