Nursing professionals have an ethical duty to ensure patient safety. According to Lavin et al. (2015), “Direct care nurses, at their core, are risk managers. They attach meaning to what is and anticipate ‘what might be'” (para. 8). As the media and patients circulate stories about the lack of safety in healthcare institutions, it is no wonder that healthcare consumers are skeptical and providers are wary. A study out of Johns Hopkins University (McMains, 2016) suggested that medical errors are the third-leading cause of death in the United States. Versel (2016) reminded us, however, that “it's not the first time someone has called medical error the No. 3 cause of death in the U.S. John T. James, founder of a group called Patient Safety America, did that in a 2013 report in the Journal of Patient Safety” (para. 2). The World Health Organization (2019) offered this perspective: “It is estimated that there is a 1 in 3 million risk of dying while travelling by airplane. In comparison, the risk of patient death occurring due to a preventable medical accident, while receiving health care, is estimated to be 1 in 300” (para. 1). In 2022, Wilsonlaw.com outlined 10 medical errors that can lead to patient deaths, many of which can be addressed by informatics and technology. They include communication issues, information flow issues, medication errors, poor organizational transfer of knowledge, misdiagnosis, delayed diagnosis, patient identification and assessment issues, staffing and workflow issues, medical equipment malfunctions, and inadequate organizational policies.
Increasing demands on professionals in complex and fast-paced healthcare environments may lead them to cut corners or develop workarounds that deviate from accepted and expected practice protocols. These deviations are not carried out deliberately to put patients at risk but rather are often practiced in the interest of saving time or preserving a usual workflow or because the organizational culture is such that risky behaviors are commonplace. Occasionally, these inappropriate actions or omissions of appropriate actions result in harm or significant risk of harm to patients. The Patient Safety Network (2022) outlined the issues created by the COVID-19 pandemic and emphasized the additional safety risks for both patients and healthcare workers. During infection surges, we faced shortages of ventilators, catheters, and personal protective equipment (PPE) supplies. Typical unit workflows, staffing (staff were infected), and communication (wearing PPE) were disrupted. In addition, ventilated patients were placed in the prone position to support lung function, and skin integrity was compromised in unusual places. Some patients also developed additional infections while hospitalized, which further compromised their illness trajectory. “As more data become available, highlighting where innovative approaches were successful at reducing the risk of adverse events during the pandemic, researchers and policymakers will need to determine if there are particular best practices that may be suitable for continued use beyond the pandemic” (para. 9).
A safety issue that results in death, permanent harm, or serious temporary harm that requires intervention is termed a sentinel event by the Joint Commission (n.d.). In 2013, the sentinel event definition was expanded to include safety issues involving anyone on the institution's premises (Patra & De Jesus, 2022). Consider the following case scenario:
A patient being treated for a potential pulmonary embolism was inadvertently given over 17,000 units of heparin because a smart pump was mis-programmed to run at 1,000 ml per hour rather than 1,000 units per hour (20 ml). (Institute for Safe Medication Practices, 2007)
The smart pump used in this scenario was equipped with dose-calculation software that compares the programmed infusion rate to a drug database to check for dosing within safe limits. This is an example of a knowledge-based clinical decision support system, which is discussed later in this chapter. This technology is particularly important when high-alert drugs, or high-hazard drugs, are being administered. In this case, however, the available dose-checking technology had been turned off, and the pump was operated in standard, rather than smart, mode. A subsequent analysis of the error event revealed that, to save time, many nurses in the institution were bypassing the safety technology afforded by the smart pump. Even though it has been quite some time since this error occurred, we continue to see alerts and safety checks being worked around, ignored, or turned off. This chapter focuses on some of the recommended organizational strategies used to promote a culture of safety and some of the specific informatics technologies designed to reduce errors and promote patient safety. Safety is everyone's responsibility.
The 2000 Institute of Medicine's report To Err Is Human is widely credited for launching the current focus on patient safety in health care. This report was followed in 2001 by the Institute of Medicine's report Crossing the Quality Chasm, which brought to national attention healthcare quality and safety. This national attention resulted in a $50 million grant by Congress to the Agency for Healthcare Research and Quality (AHRQ) to launch initiatives focused on safety research for patients. Other initiatives prompted by these seminal reports were the Joint Commission's National Patient Safety Goals (updated yearly since 2002), the National Quality Forum's adverse events and never events list (2002), the creation of the Office of the National Coordinator for Health Information Technology (ONC) to computerize health care (2004), the formation of the World Health Organization's Alliance for Patient Safety (2004), the Institute for Healthcare Improvement's (IHI's) 100,000 Lives campaign (2005) and 5 Million Lives campaign (2008), congressional authorization of patient safety organizations created by the Patient Safety and Quality Improvement Act to promote blameless error reporting and shared learning (2005), the “no pay for errors” initiative launched by Medicare (2008), and the $19 billion congressional appropriation to support electronic health records (EHRs) and patient safety (Wachter, 2010). In 2013, the Patient Safety Movement Foundation launched the Open Data Pledge and later announced three new patient safety challenges in 2016 (Patient Safety Movement, 2016). The most pressing challenges it identified-venous thromboembolism, mental health, and pediatric adverse drug events-reflect those in which patient death could be prevented with the proper protocols in place during the provision of patient care.
The AHRQ (2019) safety culture primer suggested that organizations should strive to achieve high reliability by being committed to improving healthcare quality and preventing medical errors and demonstrating an overall commitment to patient safety; that is, everyone and every level in an organization must embrace the safety culture. Key features of a safety culture identified by the AHRQ are as follows:
- Acknowledgment of the high-risk nature of an organization's activities and the determination to achieve consistently safe operations
- A blame-free environment where individuals can report errors or near misses without fear of reprimand or punishment
- Encouragement of collaboration across ranks and disciplines to seek solutions to patient safety problems
- Organizational commitment of resources to address safety concerns (para. 1)
The Committee on Patient Safety and Education of the American Society of Anesthesiologists (2022) emphasized that “[o]rganizations with strong safety cultures are not characterized by a complete absence of adverse events, but by their commitment to capturing and responding to these events, recognizing how each one gives valuable insight for further system improvement. Unsafe cultures, in contrast, can be highlighted by a lack of trust, fear of speaking up, absence of transparency, incivility, or even workplace violence” (para. 2).
An important part of the safety culture is cultivating a blame-free environment. Errors and near misses must always be reported so that they can be thoroughly analyzed to ascertain changes needed to prevent reoccurrence. All organizations can learn from mistakes and change their organizational processes or culture to ensure patient safety. These critical points are reiterated in a recent Sentinel Event Alert released by the Joint Commission (2018). The Patient Safety and Quality Improvement Act of 2005 mandated the creation of a national database of medical errors and funded several organizations to analyze these data with the goal of developing shared learning to prevent medical errors. Organizations themselves can engage in root-cause analysis or failure modes and effects analysis (FMEA), both of which are systems analyses to examine medical errors closely to determine the system processes that need to be changed to prevent similar future errors (Harrison & Daly, 2009; Patient Safety Network, 2019c). A tool for implementing root-cause analysis, which was developed by the U.S. Department of Veteran's Affairs National Center for Patient Safety (n.d.), had three goals: to determine “what happened, why did it happen and how to prevent it from happening again” (para. 4). Everyone is encouraged to submit actual medical errors and/or patient safety issues to the Patient Safety Network (n.d.). Similarly, the IHI has a website dedicated to FMEA. “Failure Modes and Effects Analysis (FMEA) is a systematic, proactive method for evaluating a process to identify where and how it might fail, and to assess the relative impact of different failures in order to identify the parts of the process that are most in need of change” (IHI, n.d.-b, para. 1). This powerful tool can be viewed at www.ihi.org/resources/Pages/Tools/FailureModesandEffectsAnalysisTool.aspx. The discussion accompanying the systems approach advocated by the Patient Safety Network (2019c) emphasized “that most errors reflect predictable human failings in the context of poorly designed systems (e.g., expected lapses in human vigilance in the face of long work hours or predictable mistakes on the part of relatively inexperienced personnel faced with cognitively complex situations)” (para. 2).
If one embraces a blame-free environment to encourage error reporting, then where does individual accountability fit in? According to the AHRQ (2019), one way to balance these competing cultural values (blameless vs. accountability) is to establish a just culture in which system or process issues that lead to unsafe behaviors and errors are addressed by changing practices or workflow processes and a clear message is communicated that reckless behaviors are not tolerated. The just culture approach accounts for three types of behaviors that lead to patient safety compromises: (1) human error (i.e., unintentional mistakes), (2) risky behaviors (i.e., workarounds), and (3) reckless behavior (i.e., total disregard for established policies and procedures). The Joint Commission (2018) indicated that to encourage reporting in a just culture, leadership must use accountability assessment tools to distinguish between human error and reckless behavior: “Leadership must gradually change the culture so that the need to report and do something about a safety issue outweighs the fear of being punished” (para. 11).
We turn now to safety initiatives more specifically related to technology. A systems engineering approach to patient safety, in which technology manufacturers partner with organizations to identify risks to patient safety and promote safe technology integration, was advocated by Ebben et al. as early as 2008. They noted that human factors engineering is “[t]he discipline of applying what is known about human capabilities and limitations to the design of products, processes, systems, and work environments” and its application to system design improves “ease of use, system performance and reliability, and user satisfaction, while reducing operational errors, operator stress, training requirements, user fatigue, and product liability” (p. 327). For example, Ebben et al. described the feel of an oxygen control knob that rotated smoothly between settings, which suggested to the user that oxygen flows at all points on the knob, when in fact oxygen flowed only at specifically designated liter flow settings. Human factors engineering testing would most likely reveal this design flaw, and the setting knob could be improved to include discrete audio or tactile feedback (click into place) to the user to indicate a point on the dial where oxygen flows. Ebben et al. also emphasized that testing human use factors provides more objective safety data than the subjective responses gained from user preference testing. “Understanding how the equipment shapes human performance is as important as evaluating reliability or other technical criteria” (p. 329). A World Health Organization (2016) publication makes an important point about human factors engineering: “The overall human factors philosophy is that the system should be designed to support the work of people, rather than designing systems to which people must adapt” (p. 5). Further, this publication cautions that issues with information and information chaos contribute to safety incidents because clinicians are overwhelmed with information. It defined information chaos as follows:
- Information overload (i.e., too much unnecessary information)
- Information underload (i.e., missing or not enough information)
- Information scatter (i.e., information located in many different places and difficult to find)
- Erroneous or conflicting information (p. 6)
Organizations that are purchasing medical technology devices should avail themselves of shared safety data on equipment maintained by several key organizations, including the Joint Commission, the U.S. Food and Drug Administration (FDA), and the Medical Product Safety Network (www.fda.gov/medical-devices/medical-device-safety/medsun-medical-product-safety-network). Many healthcare practitioners feel that we have not made great strides in either sharing our data or accessing the available data to enhance patient safety interests. According to the WISH Patient Safety Forum (Pronovost et al., 2015), the patient safety premises that harms are inevitable, data silos (rather than shared data) are natural, and heroism is the norm “have inadvertently provided excuses for not addressing patient safety comprehensively” (p. 9). This forum also stated the following:
The belief that data silos are acceptable in healthcare settings is an irresponsible view regarding the role of data; it lacks an understanding of the current operational setting. Healthcare is a complex, multidisciplinary environment that requires collaboration and sharing of data across an integrated stakeholder community. (p. 9)
As health information technology (IT) evolves and is refined, it continues to improve patient safety. Banger and Graber (2015) stated the following:
ONC is involved in a number of initiatives in support of this goal, including plans for a new national Health IT Safety Center to coordinate these efforts. Combined with the active engagement from the private sector, there is every reason to be optimistic that health IT will continue to improve the quality and safety of health care beyond the accomplishments realized to date. (p. 10)
According to the Patient Safety Network (2019b), “busy health care workers rely on equipment to carry out life-saving interventions, with the underlying assumption that technology will improve outcomes” (para. 2). The Patient Safety Network provided the following descriptions of equipment issues:
An obstetric nurse connects a bag of pain medication intended for an epidural catheter to the mother's intravenous (IV) line, resulting in a fatal cardiac arrest. Newborns in a neonatal intensive care unit are given full-dose heparin instead of low-dose flushes, leading to three deaths from intracranial bleeding. An elderly man experiences cardiac arrest while hospitalized, but when the code blue team arrives, they are unable to administer a potentially life-saving shock because the defibrillator pads and the defibrillator itself cannot be physically connected. (para. 1)
Once the technology is integrated into the organization, biomedical engineers can become valuable partners in promoting patient safety through appropriate use of these technologies (Figure 15-1). For example, in one organization, the biomedical engineers helped to revamp processes associated with the new technology alarm systems after they discovered several key issues: slow response times to legitimate alarms and multiple false alarms (promoting alarm fatigue) created by alarm parameters that were too sensitive. Strategies for addressing these issues included improving the nurse call system by adding Voice over Internet Protocol telephones, which wirelessly receive alarms directly from technology equipment carried by all nurses, thus reducing response times to alarms; feeding alarm data into a reporting database for further analysis; and encouraging nurses to round with physicians to provide input into alarm parameters that were too sensitive and generating multiple false alarms (Joint Commission, 2013; Schlabig Williams, 2009).
Figure 15-1 User-Technology-Patient Safety Scheme
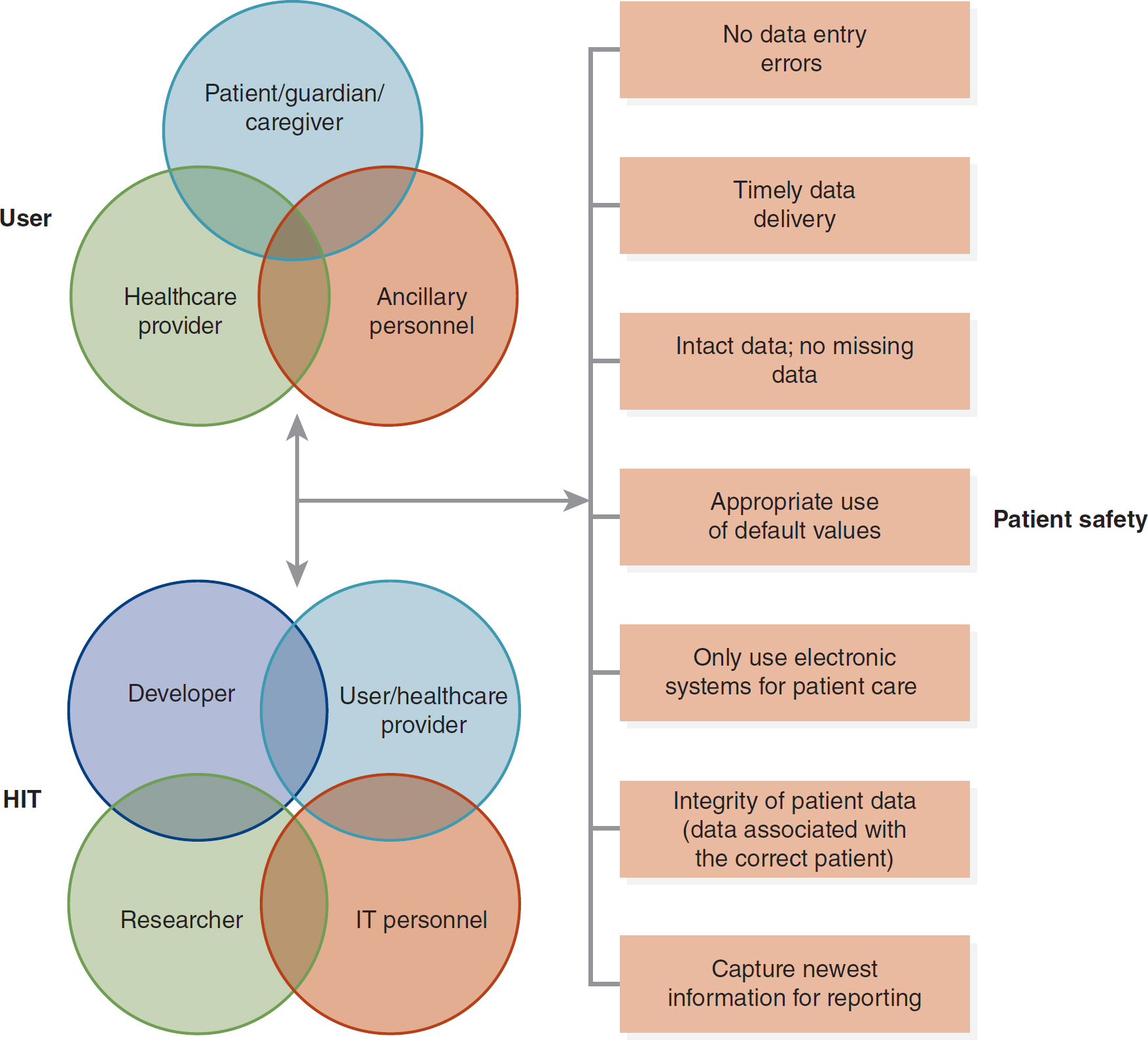
A three-part illustration outlines the user-technology-patient safety scheme.
In the first section, three circles overlap to represent the user, encompassing patient or guardian or caregiver, healthcare provider, and ancillary personnel. The second part focuses on health information technology, H I T, with four overlapping circles indicating developer, user or healthcare provider, researcher, and I T personnel. These components lead to a flow diagram of patient safety, emphasizing criteria such as the absence of data entry errors, timely data delivery, intact and complete data with no missing data, appropriate use of default values, exclusive electronic system utilization for patient care, integrity of patient data, that is, data associated with the correct patient, and capturing the latest information for reporting.
The case scenario, Well-Intentioned Providers, demonstrates how well-intentioned healthcare providers can cause harm. An audit conducted by Philips Healthcare (2013) at one of its customer sites revealed the following:
[A] Telemetry Charge Nurse was found to be receiving and responding to an average of 3.7 alarms per minute over the duration of the audit. Even allowing for minimal time to respond to each alarm, it is clear that this situation was problematic. A majority of that nurse's time was spent responding to alarms, and inevitably some were missed. (para. 1)
Goal 4 of the 2023 Hospital National Patient Safety Goals of the Joint Commission is “Make improvements to ensure that alarms on medical equipment are heard and responded to on time” (para. 4). Interestingly, this goal first appeared in the 2014 edition and has been included every year since then. Many patient monitoring and medication administration technologies are equipped with alarms to alert caregivers of potential problems. At issue is the number of alarms, especially false alarms caused, for example, by artifact or patient movement. Multiple false alarms can lead caregivers to become desensitized so that they ignore or even turn off alarm systems. Some strategies suggested by the Joint Commission include developing policies and procedures to establish who can set or change alarm parameters, when an alarm can be disabled, and who is responsible for responding to alarms.
| Case Scenario: Well-Intentioned Providers |
|---|
| Even well-intentioned healthcare providers can cause harm. Consider what should have been done differently in the following case scenario.
Laura, a 25-year-old woman, arrived at the ER complaining of chest pain. She has two young children at home, a 6-year-old boy and a 4-year-old girl. She stated that she has been experiencing severe fatigue and fluttering in her chest for weeks but felt that she needed rest and it was probably nothing. Today, she had the fluttering with chest pain, and even her teeth and jaw hurt. This scared her, so she decided to go to the hospital. However, she had to wait 2 hours for her mother to arrive to watch the children. Her husband is on a business trip and will not be returning for 4 days. The initial ECG revealed normal sinus rhythm, and all lab values were normal. The emergency room physician decided to keep her for observation and sent her to the telemetry unit.
Laura was moved to telemetry and, as she stated, “wired for sound.” The nurse described the equipment and told her that in addition to all of the monitoring equipment, they would check her vital signs every hour as well. The nurse no sooner returned to the nurse's station when Laura's cardiac monitor alerted her that Laura was experiencing severe bradycardia (i.e., heart rate of less than 40 beats per minute). When the nurse arrived at Laura's bedside, she found Laura sound asleep. She woke her gently and told her that her monitor was alarming and that she was going to check her. Laura stated that she felt tired and was enjoying the peaceful sleep. Laura's vital signs were fine and her heart rate was 72 beats per minute. The nurse reset the monitor, by which point Laura had already fallen back to sleep. The monitor alarmed the same way three more times within the next hour. Each time, the nurse woke Laura and everything was fine. The nurse decided to contact the resident. While she was waiting for the resident, it alarmed twice again, but she just reset it and let Laura sleep. The resident came and examined Laura. The resident felt everything was OK and that this young mother needed her rest. The resident suggested that the nurse stop the hourly vitals; call and have the equipment examined by the biomedical department; and, in the meantime, turn the alarm off. The nurse agreed, turned off the alarm, placed a call to the biomedical technician on duty, and left a message.
The nurse had another patient who also had frequent alarms, but his corresponded to actual medical events. As a result, the nurse was spending a great deal of time with this elderly gentleman and his wife. Each time she walked by Laura's bed, the nurse noted that Laura was sleeping. She realized that it had been 2 hours since she had turned off the alarm and called the biomedical technician, so she decided to check on Laura; however, her other patient's alarm went off, and since Laura was sleeping, the nurse went to the other patient's bedside. At 4 hours after the alarm had been turned off, the biomedical technician arrived and apologized because one of the other techs called off sick in their department and they were shorthanded. The nurse explained what had happened, and the biomedical technician went to check Laura's monitoring equipment. The biomedical technician called for the nurse because the patient was unresponsive. The nurse could not wake Laura, and the monitor was showing asystole. A code was initiated, and Laura was pronounced dead 5 hours after she had arrived on the telemetry unit.
This situation was assessed by the patient safety officer and the patient safety committee. Their determination was that because the monitor was integrated and all functions ran through the same controller, the nurse did not realize she was turning off all of the monitors (e.g., pulse oximetry and blood pressure). This situation was found to be an issue with the equipment itself because the alarm settings are too close together and not clearly labeled; however, the nurse should never have turned the alarms off. With the hourly checks canceled and all the monitoring equipment silenced, Laura was not being monitored at all. Well-intentioned providers were allowing this young mother to sleep but with fatal consequences.
|
It is evident from the case scenario that we have yet to find a solution to the problem of alarm fatigue and related issues that negatively affect patient safety.
Clearly, there is more work to be done to create safety cultures in complex healthcare organizations and to reduce the incidence of errors. Many organizations are looking to informatics technology to help manage these complex safety issues by using smart technologies that provide knowledge access to users and automated safety checks and that improve communication processes. Harrison (2016) stated that “as nurse leaders in a clinical setting where smart tools are leveraged to increase the quality and safety of patient care, we have certain responsibilities to ensure safe implementation, training, and monitoring” (p. 21). To best utilize the available technology, nurse leaders and administrators must be able to use data. More and more graduate programs for nursing administrators are realizing the need for these emerging nursing leaders to be skilled in nursing informatics. These leaders must be able to use data, information, and knowledge efficiently and effectively to assess and manage their clinical settings and, ultimately, to apply these informatics skills to improve patient outcomes and the quality of patient care (Figure 15-2).
Figure 15-2 Data and Quality Connection: There are many ways to obtain data and information. The skill is in knowing how to access, select, and use the data and information, applying nursing informatics to inform practice and improve patient care.
A team of healthcare professionals uses shovels to dig up a lawn for planting plants. A team of healthcare professionals uses garden trowels to dig up a lawn for planting plants. A group of healthcare professionals utilizing garden trowels to unearth a lawn for planting plants serves as a metaphor for their efforts in excavating data and information. A healthcare professional tends to an elderly patient. A healthcare professional tends to a pediatric patient in the presence of the caregiver.
In 2016, the Government Accountability Office (GAO) selected and assessed six hospitals, from which it identified three challenges in implementing patient safety practices. The number one challenge was “obtaining data to identify adverse reactions in their own hospitals” (para. 2). Nursing informatics skills and knowledge can address this challenge.
The GAO interviewed patient safety experts and reviewed the related literature to identify three key gaps where better information could help guide hospital officials in their continued efforts to implement patient safety practices. These gaps involve a lack of “(1) information about the effect of contextual factors on implementation of patient safety practices, (2) sufficiently detailed information on the experience of hospitals that have previously used specific patient safety implementation strategies, and (3) valid and accurate measurement of how frequently certain adverse events occur” (p. 22). It is clear that implementing solid nursing informatics practices, skills, and knowledge can close these gaps.
Healthcare technologies are frequently designed to improve patient safety, streamline work processes, and improve the quality and outcomes of healthcare delivery. As we continue to look to health IT to advance patient safety initiatives, we must realize that integrating health IT presents other challenges and can add to patient safety issues. For example, Singh and Sittig (2015) stated that health IT has the “potential to improve patient safety but its implementation and use has led to unintended consequences and new safety concerns. A key challenge to improving safety in health IT-enabled healthcare systems is to develop valid, feasible strategies to measure safety concerns at the intersection of health IT and patient safety” (p. 226).
Although technology may certainly help to prevent or reduce errors, one must always remember that technology is not a substitution for safety vigilance by the healthcare team in a safety culture. Harrison (2016) stated that “[p]atient safety should always be at the center of the design and adoption of any technology introduced into patient care settings. Technology that's designed to improve patient safety is only as good as the person using the device. It doesn't replace critical thinking, solid nursing practice, and careful patient monitoring” (p. 21).
The Wired for Health Care Quality Act of 2005 began a series of funding streams to promote health IT and sharing of its best practices and to help organizations implement health IT (Harrison & Daly, 2009). Many early adopters opted to focus technology and safety initiatives on medication ordering and administration processes. Medication errors are the most frequent and visible errors because the medication administration cycle has many poorly designed work processes with several opportunities for human error. Thus, computerized provider order entry (CPOE), automated dispensing machines, smart pump technologies for IV drug administration, and barcode medication administration (BCMA) frequently preceded the adoption of the EHR in many institutions because of the comparatively lower costs associated with implementing these technologies. In an ideal world, the EHR would be adopted concurrently as part of an interoperable health IT system. In the early EHR systems, clinicians were prompted by electronic alerts to remind them of important interventions that should be part of the standard of care, but these alerts tended to be generalized and not patient specific-for example, “Did you check the allergy profile?” or “Has the patient received a pneumonia immunization?” These early alert and care reminders are now evolving into more sophisticated clinical decision support (CDS) systems to promote accurate medical diagnoses and to suggest appropriate evidence-based medical and nursing interventions based on patient data.
With the addition of triggers to detect adverse events, diagnostic errors, adverse drug events, hospital-acquired infections, and delays in diagnoses have been identified. In addition to the National Patient Safety Goals issued by the Joint Commission, which reference general safety hazards, other safety organizations and institutes provide information on top safety concerns. Cheney (2019) reported on the list of top 10 medical technology hazards of 2020 identified by the ECRI. The ECRI was founded in 1968 as the Emergency Care Research Institute and designated by HHS in 2008 as a Patient Safety Organization; in 2020, it affiliated with the Institute for Safe Medication Practices and was rebranded as ECRI, “the most trusted voice in healthcare” (ECRI, n.d.). The medical technology hazards identified by the ECRI were as follows:
- Surgical staplers-staple line failures or misapplication
- Point-of-care ultrasound-issues with user training, documentation and data archiving
- Infection risks from sterile processing-especially in medical and dental offices and ambulatory settings
- Hemodialysis risks with central venous catheters in the home health setting-risks include infection, clotting or hemorrhage
- Surgical robotic procedures-limited tactile feedback for forces exerted on tissue may result in injury
- Alarm, alert, and notification overload-numerous alarms may cause a clinically significant issue to be missed
- Cybersecurity risks in the home health setting-increased vulnerabilities associated with remote monitoring and network connected medical technologies
- Missing implant data for MRI scan patients-implants can heat, move or malfunction when exposed to MRI's magnetic field
- Medication errors from dose timing discrepancies in electronic medical records-discrepancies between dose timing intended by the provider and nursing workflow
- Loose nuts and bolts in medical devices-devices can tip, fall or collapse if not properly maintained (Cheney, 2019, para. 7)
Many of these issues can be prevented or detected in their early stages using informatics technologies, although we do see continued struggles with the same safety issues over several years. Other technologies designed specifically to promote patient safety include wireless technologies for patient monitoring, clinician alerts, point-of-care applications, apps, and radio-frequency identification applications. Each of these technologies is reviewed here, and the chapter concludes with a section that discusses future technologies for patient safety.
Technologies to Support the Medication Administration Cycle
The steps in the medication administration cycle (i.e., assessment of need, ordering, dispensing, distribution, administration, and evaluation) have been relatively stable for many years. Each of the steps depends on vigilant humans to ensure patient safety, which resulted in the five rights of medication administration: (1) the right patient, (2) the right time and frequency of administration, (3) the right dose, (4) the right route, and (5) the right drug. Human error can be related to many aspects of this cycle. Distractions, unclear thinking, lack of knowledge, short staffing, and fatigue are a few of the factors that cause humans to deviate from accepted safety practices and commit medication errors. Integration of technology into the medication administration cycle promises to reduce the potential for human errors in the cycle by performing electronic checks and providing alerts to draw attention to potential errors.
CPOE is an electronic prescribing system designed to support physicians and nurse practitioners in writing complete and appropriate medication and care orders for patients. When CPOE is part of an EHR with a CDS system, the medication order is electronically checked against specific data in the patient record to prevent errors, such as ordering a drug that might interact with a drug the patient is already taking, ordering a dose that is too large for the patient's weight, or ordering a drug that is contraindicated by the patient's allergy profile or renal function. Because it is impossible for and unreasonable to expect a clinician to remember the numerous drugs that require a dose adjustment in the case of renal dysfunction, for example, safe dosing parameters are provided by the CDS associated with a CPOE (Patient Safety Network, 2019a). In a stand-alone CPOE system without a CDS system, the medication orders are simply checked by the computer against the drug database to ensure that the dose and route specified in the order are appropriate for the medication chosen. Specific benefits of a CPOE system include the following:
- Prompts that warn against the possibility of drug interaction, allergy, or overdose
- Accurate, current information that helps physicians keep up with new drugs as they are introduced into the market
- Drug-specific information that eliminates confusion among drug names that look and sound alike
- Reduced healthcare costs caused by improved efficiencies
- Improved communication among doctors, nurses, specialists, pharmacists, other clinicians, and patients
- Improved clinical decision support at the point of care (Steele & DeBrow, n.d.)
CPOE solves the safety issues associated with poor handwriting and unclear or incomplete medication orders. Orders can be entered in seconds and from remote sites, thereby eliminating the use of verbal orders, which are especially subject to interpretation errors. Orders are then transmitted electronically to the pharmacy, thus reducing the potential for transcription errors that are commonly encountered in the former paper-based system, such as lost or misplaced orders, delayed dosing, or unreadable faxes. As with any technology integration, introduction of CPOE is associated with a resistance to change in the typical workflow, a learning curve to gain proficiency, and users must learn to trust the system.
The verification and dispensing functions of the pharmacy can also be assisted by technology. The pharmacist begins by verifying the allergy status of the patient and the medication reconciliation information to ensure that the new medication is compatible with other medications in the care regimen. This verification function is computer based, and the medication order is electronically checked via the knowledge database. If the order is verified as safe and appropriate, the pharmacist proceeds to the dispensing process. Barcode medication labeling at a unit-dose level was mandated by the FDA in 2004, with targeted compliance to be achieved by 2006. A barcode is a series of alternating bars and spaces that represent a unique code that can be read by a special barcode reader. Barcode technology spans both the medication dispensing and the administration steps in the medication administration cycle. In the pharmacy, the barcode helps to ensure that the right drug and the right dose are dispensed by the pharmacy. Medications that are labeled with barcodes can also be dispensed by robots capable of reading the codes or automated dispensing machines. In this way, barcode technology helps with the processes of procurement, inventory, storage, preparation, and dispensing (University of Rochester Medical Center, n.d.).
The processes of drug storage, dispensing, controlling, and tracking are easily carried out via automated dispensing cabinets (ADCs; also known as automated dispensing machines, unit-based cabinets, automated dispensing devices, and automated distribution cabinets). These devices have benefits for both the user and the organization, specifically in the areas of access security (especially with narcotics administration tracking), safety, supply chain, and charge functions (Institute for Safe Medication Practices, 2019). However, as Rhodes and McCarthy (2019) pointed out, errors are still possible when the override function is used to access medications from an ADC in emergent situations. They suggest that when overriding the system, the user should be forced to enter an indication for use to allow the system to detect an inappropriate medication selection from an alphabetical list. They provided two examples, one where diazepam instead of diltiazem was selected and another where vecuronium instead of Versed was selected. In the first case, if the user had been required to enter an indication, such as cardiac disturbance, they would have been prevented from selecting diazepam. Likewise, if an indication for sedation had been entered, then the ADC would have prevented dispensing a neuromuscular blocking agent.
Applications (apps), or mobile apps, are being used by and prescribed for patients. The apps used for patient education can engage and inform patients; however, apps must be used judiciously. If apps are prescribed for patients, then it is the healthcare personnel's responsibility to educate the patient and/or family on their proper use. It is important that patients and their families understand the benefits and risks of using the app as well as how to receive help when needed. If data are being exchanged, patients must comprehend what data will be collected and where, when, and with whom they will be shared. The University of Florida libraries developed this website listing patient education apps: https://guides.uflib.ufl.edu/mobilehealth/patienteducation.
There are apps for healthcare personnel as well. iScrub is an app that is used to monitor hand hygiene, which could help prevent healthcare-associated infections (Soft112, n.d.). The Patient Safety Manual for Android was designed as a resource to treat patients quickly, safely, and effectively (APKPure, 2019). Other popular apps include Pepid, Pill Identifier, Medical Spanish, Pedi STAT, Critical Care ACLS Guide, Medscape, Headspace, Pocket Lab Values, Pocket Pharmacist, and Epocrates (Morris, 2023). Apps will continue to be used by providers and patients, so all healthcare personnel must assume the responsibility of making sure that the apps are both appropriate to use and used appropriately.
Radio-frequency identification (RFID) technology is rapidly gaining a foothold in healthcare technology and may soon be used in the medication administration cycle. Although more expensive than bar coding for packaging, the RFID tags are reprogrammable, and issues associated with barcode printing imperfections and scanner resolution can be mitigated (Vecchione, 2015). As discussed later in this chapter, RFID technologies may also be an important component of a medication compliance system for patients.
BCMA systems help to ensure adherence to the five rights of medication administration. Whether BCMA is part of the larger EHR or a freestanding electronic medication administration system (eMAR), barcode technology provides a system of checks and balances to ensure medication safety. The nurse begins by scanning their badge, thereby logging in as the person responsible for medication administration. Next, the barcode on the patient's identification bracelet is scanned, which prompts the electronic system to pull up the medication orders. Next, the barcode on each of the medications to be administered is scanned. This technology check ensures that the five rights of medication administration are met. If there is a discrepancy between the order and the medication that was scanned or a contraindication for administration, an alert is generated by the system. For example, in an EHR system with CDS, the nurse may be prompted to check the most recent laboratory results for electrolytes before administering a potassium supplement. In a freestanding eMAR without CDS or EHR links, if the medication orders have recently been changed, the nurse is alerted to the change. When an alert is generated, the nurse must chart the action taken in response to that alert. For example, an early dose might need to be given if the patient is leaving the unit for a diagnostic test.
Despite the promising advances in patient safety afforded by this technology, it is not fail-safe. Medications that are labeled individually by the in-house pharmacist increase the potential for human error if the medication is given an incorrect barcode, such as one signifying a wrong dose or even the wrong medication. Many hospitals are now purchasing medications labeled by the manufacturer. Van der Veen et al. (2020) studied workarounds (defined as temporary practices deviating from normal workflow expectations) associated with BCMA systems. In the 5,793 medication administrations, they observed workarounds in 62.7% of the administrations. “Procedural workarounds (as not scanning at all) were most common (n = 1,307, 36%). Other workarounds observed were patient scanning-related (as no barcode wristband on the patient; n = 1,017, 28%) and medication scanning-related (including scanning before actual administration of medication, scanning medication for more than one patient at a time, and ignoring computer or scanner alerts; n = 400, 11%)” (p. 5). They concluded that nurse-to-patient ratios, timing of the scheduled administrations, and considerations of nurse workload were all modifiable factors that could improve compliance with this important patient safety technology. Strudwick et al. (2018) reported similar types of workarounds with BCMA that contributed to medication errors. They suggested that careful attention to nurse workflows was critical to safeguarding the proper use of BCMA.
Smart pump technologies are designed for safe administration of high-hazard drugs and reducing adverse drug events during IV medication administration. Smart pumps have software that is programmed to reflect the facility's infusion parameters and a drug library that compares normal dosing rates with those programmed into the pump. Discrepancies generate an alarm that alerts the clinician to a safety issue. A soft alarm can typically be overridden by a clinician at the bedside, but a hard alarm requires the clinician to reprogram the pump so that the dosing falls within the facility's IV administration guidelines for the drug to be infused. All alarms generated by the smart pump are tracked along with the clinician's responses to them (Cummings & McGowan, 2011; Hulen, 2013). Smart pumps can be seamlessly integrated into BCMA systems, and data can be fed directly into the EHR. The IHI (n.d.-c) recommends the following steps to ensure safe implementation of smart pump technology:
- Prior to deploying these pumps, standardize dosing units for a given drug (for example, agreeing to always dose nitroglycerin in terms of mcg/min or mcg/kg/min but not both). Asking a nurse to choose among several dosing units increases the risk of selection error.
- Prior to deploying these pumps, standardize drug nomenclature (for example, agreeing to always use the term KCl, but not Potassium chloride, K, Pot Chloride, or others). Asking the nurse to remember and choose among several possible drug names increases the risk of selection error.
- Perform a Failure Modes and Effects Analysis (FMEA) on the deployment of these devices.
- Ensure that the concentrations, dose units, and nomenclature used in the pump are consistent with that used on the Medication Administration Record (MAR), the pharmacy computer system, and the electronic medical record.
- Meet with all relevant clinicians to come to agreement on the proper upper and lower hard and soft dose limits.
- Monitor overrides of alerts to assess if the alerts have been properly configured or if additional quality intervention is required.
- Be sure the “smart” feature is utilized in all parts of the hospital. If the pump is set up volumetrically in the operating room but the “smart” feature is used in the ICU, an error may occur if the pump is not properly reprogrammed.
- Be sure there are upper and lower dose limits for bolus doses, when applicable.
- Engage the services of a human factors engineer to identify new opportunities for failure when the pumps are deployed.
- Identify a procedure for the staff to follow in the event a drug must be given which is either not in the library or when its concentration is not standard.
- Deploy the pump in all areas of the hospital. If a different pump is used on one floor and the patient is later transferred, this will create new opportunities for failure. Also, there may be incorrect assumptions about the technology available to a given floor or patient.
- Consider using “smart” technology for syringe pumps as well as large volume infusion devices. (para. 12)
Nurses must always engage in best practices and follow all patient safety practices. There is no substitute for nursing assessment of patients as a key safety tool.
A CDS can enhance the medication administration cycle by promoting safety and improving patient outcomes. The CDS is guided by targeted information delivery, ensuring that the five rights of CDSs are implemented: the right information provided to the right person in the right format through the right channel at the right time in the workflow. For example, during medication selection, a CDS helps a clinician select an appropriate medication based on client data, such as clinical condition, weight, renal function, concurrent medications, and cost. This system ensures that the medication order is complete by performing checks for drug interactions, duplications, or allergy contraindications and ensures that the right dose and the right route are specified. During the verification and dispensing phase of the medication administration cycle, the CDS provides double checks for interactions, allergies, and appropriate dose orders. Consideration is also given to potential infusion pump programming issues, incompatibilities during infusion, and proper notation and dispensing when portions of a dose must be wasted. During the administration phase, the CDS assists with patient identification and current assessment parameters (i.e., blood pressure and glucose level) that may contraindicate the use of the medication at that point in time. In addition, checks for interactions with foods or other medications and timing and monitoring guidelines are provided to the clinician administering the medication. The CDS has patient education guidelines and printable handouts to assist clinicians in educating patients about their medications. The monitoring functions of the CDS provide a structured data reporting system to track side effects and adverse events across the population. Because smart pumps are often used for infusion of high-hazard drugs, systems are being developed to promote EHR bidirectional interoperability between the pump and the medical record. “With this level of bidirectional interoperability, infusion parameters are wirelessly transmitted from the EHR to prepopulate the smart infusion pump, and infusion data is also wirelessly sent back into the EHR” (Institute for Safe Medication Practices, 2017, para. 1).
Several promising technologies may become more widely available in the future to assist patients with medication compliance after discharge. For example, caps of pill bottles may contain RFID tags that monitor and collect data on when the bottle is opened or that contain flashing time reminders when a dose is due (Blankenhorn, 2010). Mason et al. (2022) point out that opening the pill bottle is a proxy for taking the medication and does not ensure adherence. Bluetooth-enabled smart inhalers track asthma medication compliance and whether the device was used properly (Pharmacy Alternatives, n.d.). Licholai (2019) listed several technologies that use smartphones to promote adherence: Medisafe, MyMeds, Care4Today, MedMinder, and AdhereTech. InPen is a smart insulin pen that couples with a smartphone to calculate insulin dosages and track injections (Medgadget, 2016a), PillDrill helps people adhere to a medication schedule and includes a “mood cube” to track how patients are feeling (Medgadget, 2016b), and Proteus smart pills have sensors attached to medications to track when the pill is actually ingested (Medgadget, 2015). In addition, several technologies track adherence and then issue gift card rewards (Licholai, 2019). These are just a sampling of the newer technologies for medication adherence; more are expected to emerge in the future. However, these technologies add costs to the medications that might preclude widespread adoption.
Additional Technologies for Patient Safety
CDS systems have safety uses beyond the medication administration cycle. The robust data collection and data management functions of a CDS embedded in an EHR help to ensure quality approaches to patient health challenges based on research evidence and clinical guidelines. A CDS may also ensure cost effectiveness by alerting clinicians to duplicate testing orders or suggesting the most cost-effective diagnostic test based on specific patient data (Charles, 2018).
Early uses of a CDS were limited to pop-up alerts that reminded clinicians of specific interventions, such as flu/pneumonia shots, diet counseling for those with abnormal body mass indexes, or smoking cessation interventions. Newer systems reserve alerts for critical situations, such as drug interactions/allergies and impending issues such as sepsis or cardiac failure. Limiting alerts that demanded action on the part of clinicians has helped to avoid alarm fatigue and support clinical workflow. Now CDSs are designed to provide information when requested by giving clinicians access to searchable databases and clinical guidelines. CDSs are designed to deliver the following:
- the right information (evidence-based guidance, response to clinical need)
- to the right people (entire care team-including the patient)
- through the right channels (e.g., EHR, mobile device, patient portal)
- in the right intervention formats (e.g., order sets, flow-sheets, dashboards, patient lists)
- at the right points in workflow (for decision making or action) (Bresnick, 2017, para. 20)
Stay tuned for the increased use of more sophisticated CDSs that are based on artificial intelligence and machine learning to analyze data and make predictions and care recommendations based on diagnoses and trending data (Charles, 2018).
The prompts and instructions provided to the clinician by the CDS system are detailed and designed to be easy to navigate. Implementation of a CDS has the potential to optimize care by ensuring that all the details of a patient's health issues are presented to the clinician for management, thereby promoting individualized approaches to the total health of the patient based on best available evidence and clinical guidelines (HealthIT.gov, n.d.). According to the Centers for Medicare and Medicaid Services (n.d.),
CDS is not simply an alert, notification, or explicit care suggestion. CDS encompasses a variety of tools including, but not limited to:
- Computerized alerts and reminders for providers and patients
- Clinical guidelines
- Condition-specific order sets
- Focused patient data reports and summaries
- Documentation templates
- Diagnostic support
- Contextually relevant reference information
These functionalities may be deployed on a variety of platforms. (para. 2)
RFID technologies have both supply chain and patient care applications to patient safety. An RFID system contains a tag affixed to an object or a person that functions as a radio-frequency transponder and provides a unique identification code, a reader that receives and decodes the information contained on the tag, and an antenna that transmits the information between the tag and the reader. When RFID tags are embedded in patient identification bracelets, they can help with patient tracking during procedures and testing or function as part of the EHR to communicate pertinent information to clinicians at the bedside. RFIDs may eventually be part of the medication administration process, which would replace barcode technologies. They can be used to track medical supplies and equipment, thereby reducing staff time in locating such items. They may also be embedded into surgical supplies to automate supply-counting procedures, thereby reducing the likelihood that sponges or tools will be erroneously left in a patient. RFIDs may also reduce the likelihood of the never events of wrong-patient, wrong-site surgical procedures (FDA, 2018; Patient Safety Network, 2019d; Revere et al., 2010). RFIDs used in the medication supply chain protect patients by reducing the potential that a counterfeit medication might be inadvertently introduced into the supply and providing for efficient medication recalls. Potential terrorist manipulation of the medication supply is also thwarted by RFID supply chain tracking technology. Blood and blood products can be efficiently tracked by RFIDs because specialized tags can detect temperature fluctuations and therefore ensure that the blood or blood product was stored at the optimal temperature for safe administration; however, RFID technology will probably not replace barcodes in blood banking due to the cost (Wray, 2016).
Smart rooms are also being used in healthcare facilities to better engage patients and families in the hospital experience. As a caregiver enters the room, the RFID tag on their name badge announces to the patient on a monitor (typically mounted on the wall in the patient's line of sight) exactly who has entered the room and triggers need-to-know data based on caregiver status to be displayed on the monitor in the room, thus complying with the Health Insurance Portability and Accountability Act (HIPAA). For example, when a dietary aide enters the room, only dietary information is displayed; when a physician or nurse enters, all the pertinent medical data from the EHR are available. Clinicians can review patient data in real time and chart care at the bedside using touch screen technology, thereby increasing productivity. One caution, however, is that patients have to consent to family members and visitors to be able to view information or they will need to step out as the caregiver displays information. Some smart room technologies include workflow algorithms to alert clinicians as they enter the room about procedures that need to be implemented for the patient and can track individual clinician efficiency and effectiveness by aggregating data over time (Foley, 2017; Heath, 2020; Sharbaugh & Boroch, 2010). One of the disadvantages of smart room technology is the cost of outfitting a room with such technologies. More recently, hospitals are implementing an Internet of Things (IoT) device located at the bedside that allows the patient to use voice commands to control the room (i.e., temperature and lighting), search for health-related information, and improve communication with clinicians (Bengfort, 2017).
Smart beds, which provide continuous rotation to prevent pressure ulcers, sense when a patient at risk for a fall leaves the bed, and continuously monitor vital signs, are also being implemented (Dubinsky, 2017). Mesko (2017) described several additional innovations that may be implemented in the near future. They include robots to clean rooms efficiently and effectively, flat-screen TVs run by mobile devices for entertainment and patient education, prompts asking patients to record their pain levels on mobile devices, and the ability of clinicians to project three-dimensional images in the room to explain diagnostic findings and treatment plans.
Other technologies for improving patient monitoring include wearable technology and wireless area networks, variously called body area networks or patient area networks. The technologies provide the ability to wear a small, unobtrusive monitor that collects and transmits physiological data via a cell phone to a server for clinician review. Although most of these technologies are designed for monitoring patients with chronic diseases, they also have safety implications because they help to identify early warning physiological signs of impending serious health events (California Healthcare Foundation, 2013; Kosir, 2015). An early innovation was the wireless chip on a disposable Band-Aid with a 5- to 7-day battery, which was developed to monitor the patient's heart rate and electrocardiogram, blood glucose, blood pH, and blood pressure and allowed for the collection of important clinical data outside the hospital (Miller, 2008). Wearable stress-sensing monitors detect electrical changes in the skin that may signal increased stress in autistic children who are unable to communicate an impending crisis; caregivers are alerted to the potential crisis via wireless transmission and can intervene to reduce the stress and prevent the crisis (Murph, 2019). Other new technologies promise to aid in home monitoring of an elder's movement, sleep, fall detection, health data, and/or medication data, such as Caregiver Smart Solutions, Forma SafeHome, SenSights, and Vayyar Care (Orlov, 2020). Smart fabrics with monitoring technology built in are one of the latest developments in wearable technology. Hospital gowns made with smart fabric could provide vital signs monitoring, be customized by type of patient (e.g., cardiac), or be programmed to deliver medication via the skin. Continuous monitoring via wearable technology versus intermittent vital sign checks allows for the immediate identification of decline and the need for intervention in hospitalized patients. Wearable technologies also provide important insights into health status in the community. While early technologies may have contributed to patient anxiety related to inaccurate data, the evolution of wearables promises accuracy, early detection, and timely intervention. As McGinnis and McGinnis (2022) stated, “The digital medicine revolution is at the cusp of transforming biomedical research and clinical practice by providing unprecedented access to ecologically valid health data and the ability to deliver personalized, data-informed, health-improving interventions directly to the patient wherever and whenever needed” (para. 2).
Robotics technologies are also being increasingly tested for safety and efficiency uses. Robotics has been used in minimally invasive surgery for some time; as with most technologies, there are risks and rewards (Kirkpatrick & LaGrange, 2016). Surgical robots continue to evolve, and newer technologies feature a three-dimensional high-definition vision system and precision motion controlled by the surgeon, which enables them to be used in various types of precise surgeries (Medical Education Network, 2021; Sniderman, 2018). Robotic exoskeletons are used for gait rehabilitation after serious injuries (Sniderman, 2018). Robobear, a robot designed in Japan to assist with patient lifting, provides increased safety for both patients and clinicians (RIKEN, 2015). Laser-guided robots can move around hospitals performing such routine functions as emptying and disposing of trash, cleaning rooms, delivering supplies and meals, and dispensing drugs. Robots are now able to draw blood and collect vital signs in hospitals and are used for telehealth visits and to enhance home care; they are also created to serve as companions for the aging (typically, as cats or dogs) and for those with autism or mental health challenges. Nanorobots are being tested for use as digital pills to repair stomach issues and retrieve swallowed items (Medical Education Network, 2021). Personalized health care or precision medicine, which tailors treatment to the specific genetic characteristics of individual patients and challenges patients to be more accountable for their own health, is rapidly advancing as vendors develop targeted therapies. Its potential effect is aligned with both quality and safety. Researchers pursue specific clinical trials and make certain that the data, information, and knowledge generated are captured and disseminated. Building, analyzing, and sharing large sets of medical data allow for both innovation and targeted treatments (Medline Plus, 2022).
The International Medical Informatics Association (n.d.) Health Informatics for Patient Safety Working Group emphasized that health information systems should be designed specifically to promote and enhance patient safety and that instances of technology induced safety hazards should be widely shared to improve safety.

