AUTHORS: Michael Lawrenz Co, MD, MSc and Daniel R. Frisch, MD
Typical atrial flutter is the term commonly applied to the atrial macroreentrant circuit that circulates around the tricuspid annulus in the right atrium. The critical isthmus of the circuit is the tissue between the inferior vena cava and the tricuspid annulus, and a more precise name for this arrhythmia is cavotricuspid isthmus-dependent atrial flutter, or CTI flutter. Because of its anatomic and physiologic stability, the result is regular atrial depolarizations, typically at a rate of 250 to 350 beats/min. Regular macroreentrant atrial arrhythmias at this rate that do not use the CTI are referred to as atypical atrial flutter. Because of the stability of atrial flutter, conduction through the atrioventricular node (AVN) is often predictable at a common mathematical denominator. For example, when the flutter rate is 300 beats/min, 2:1 conduction results in a ventricular rate of 150 beats/min. By extension, 3:1 conduction results in a ventricular rate of 100 beats/min, 4:1 in a rate of 75 beats/min, and 5:1 in a rate of 60 beats/min. If the regular atrial impulses conduct at a variable rate through the AVN, the result may be an irregular QRS pattern. However, a common denominator atrial interval is often present.
Table 1 summarizes the different types of atrial flutter and distinguishing features on scalar electrocardiography.
| ||||||||||||||||
TABLE 1 Characteristics of Different Types of Atrial Flutter and Distinguishing Features on Scalar Electrocardiography
| Type | Reentrant Circuit | ECG Pattern | Lead V1/V6 |
|---|---|---|---|
| Typical counterclockwise | Tricuspid annulus dependent on the CTI | Sawtooth flutter wave; negative in II, III, and aVF | Positive V1, negative V6 |
| Typical clockwise | Tricuspid annulus dependent on the CTI | “Inverse sawtooth”; positive and often notched in II, III, and aVF | Broad and negative in V1 (often notched)Positive in V6 |
| Lower loop reentry | CTI | Usually similar to typical counterclockwise CTI flutter except subtle loss of terminal positive deflection in leads II, III, and aVF | Usually similar to typical counterclockwise |
| Upper loop reentry | Superior vena cava and upper crista terminalis | Similar to typical clockwise flutter | Similar to typical clockwise flutter |
| Right atrial free wall | Around areas of scar in lateral or posterior right atrium (caused by previous atrial surgery or spontaneously) | Variable | Typically negative or biphasic with terminal negative deflection in V1 |
| Septal atrial flutter | Atrial septum, typically after previous surgery | Variable | Usually biphasic or isoelectric in V1 |
| Mitral annular flutter | Around mitral annulus, often slow zone of block around PV interval; frequently occurs in setting of left atrial surgery or ablation | Variable; I, III, and aVF, often positive but low amplitude | Usually positive in V1 (or rarely isoelectric) and often broad |
| Postatrial fibrillation ablation/maze flutter | Variable; circuit involves previous ablations or scar in left atrium | Variable | Variable |
aVF, Augmented vector foot; CTI, cavotricuspid isthmus; PV, pulmonary vein.
From Zipes DP: Braunwald’s heart disease, a textbook of cardiovascular medicine, ed 11, Philadelphia, 2019, Elsevier.
- Atrial flutter is the second most common sustained atrial tachyarrhythmia after atrial fibrillation, with an estimated 200,000 new cases annually in the U.S.
- Atrial flutter is common in patients with congestive heart failure, chronic obstructive pulmonary disease (COPD), pulmonary vascular disorders such as pulmonary embolism, or during the first week after open heart surgery.
- Atrial flutter occurs more frequently with advancing age (5/100,000 age <50 vs. 587/100,000 age >80 yr) and 2.5 times more frequently in men than in women.
- Patients taking antiarrhythmics for chronic suppression of atrial fibrillation may present with atrial flutter.
- Atrial flutter is typically seen in patients with underlying structural heart disease and is uncommon in children or young adults without congenital heart disease.
- More than 50% of patients with atrial flutter will develop atrial fibrillation in 3 yr, and more than 80% will develop atrial fibrillation within 5 yr. This is important when considering treatment options for atrial flutter, especially anticoagulation.
Historically, the Wells classification designated atrial flutter as type I and type II. However, it is now recognized that tachycardias satisfying either of the definitions for type I or type II can be caused by reentrant circuits or by rapid focal atrial tachycardia, and this classification is infrequently used. Designating atrial flutter based on whether or not it is CTI dependent is more useful because of the management options (i.e., ablation). Type I CTI-dependent atrial flutter, also known as common atrial flutter or typical atrial flutter, has an atrial rate of 250 to 350 beats/min. The reentrant loop circles the tricuspid valve in the right atrium, passing through the CTI, a body of fibrous tissue in the lower atrium between the inferior vena cava and the tricuspid valve. CTI flutter can revolve around the tricuspid annulus in either direction (counterclockwise or clockwise) when viewing the tricuspid annulus en face.
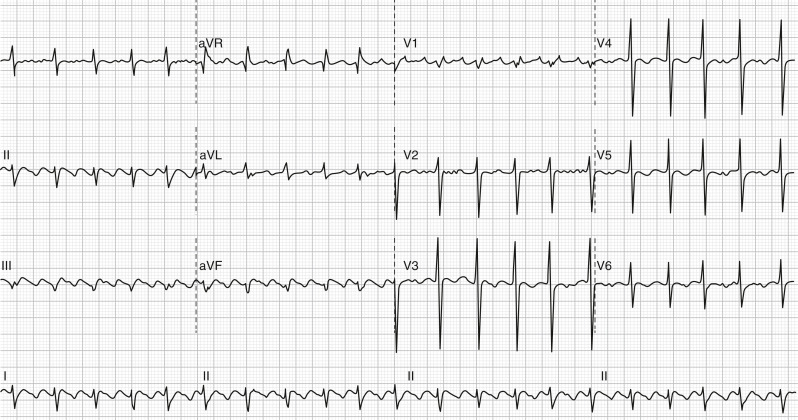
- Counterclockwise atrial flutter is the more common type (∼75%). The flutter waves are “sawtooth” and negative on the surface ECG leads II, III, and aVF; positive in V1; and negative in V6 (Fig. E1).
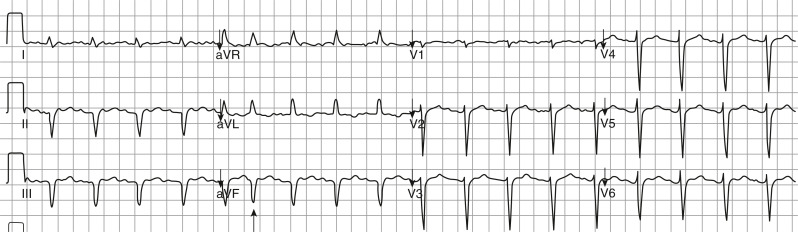
- Clockwise atrial flutter is less common (∼25%): The reentry loop cycles in the opposite direction; thus the flutter waves are upright in leads II, III, and aVF; negative in V1; and positive in V6 (Fig. E2).
- Atypical atrial flutter is defined by absence of CTI dependence and may occur in patients with prior cardiac surgery, congenital heart disease, or prior radiofrequency ablation (especially after left atrial ablation for atrial fibrillation) or may be idiopathic. One ECG feature is the lack of discordance of the flutter wave polarity between the inferior leads (leads II, III, and aVF) and V1. Flutter circuits in the left atrium (such as mitral annular flutter) often have upright flutter waves in all precordial leads.
Figure E1 Counterclockwise atrial flutter with 2:1 atrioventricular conduction.
Note the negative flutter waves in leads II, III, and F, positive in V1 and negative in V6. The second flutter wave can be seen overlying the QRS in the inferior leads and at the end of the QRS in lead V1. aVF, Augmented vector foot; aVL, augmented vector left; aVR, augmented vector right.
Figure E2 Clockwise atrial flutter with predominant 2:1 atrioventricular conduction.
Note the positive flutter waves in leads II, III, and F, negative in V1 and positive in V6. The overall ventricular rate is slower than in Fig. E1 due to a slow flutter rate of approximately 200 bpm. One beat is conducted 1:1 (arrow). aVF, Augmented vector foot; aVL, augmented vector left; aVR, augmented vector right.
- Age-related degenerative changes
- Rheumatic heart disease
- Congenital heart disease
- Left ventricular dysfunction or congestive heart failure
- Acute myocardial infarction (rarely)
- Thyrotoxicosis
- Pulmonary embolism
- Mitral valve disease
- Cardiac surgery
- Chronic obstructive pulmonary disease
- Obesity
- Pericarditis
- Pulmonary hypertension
- Antiarrhythmic therapy use in patients with atrial fibrillation
- Other causes of right atrial enlargement
- Systemic infections (such as COVID-19)1