Workup
- The World Health Organization (WHO) diagnostic criteria for various forms of mastocytosis are outlined in Tables E1, Table E2, and Table E3. Smoldering systemic mastocytosis (SSM) is the 2016 classification now defined by two or more “B” findings (Table E4).
- Serum tryptase: Approximately 20% of cases have serum tryptase less than the WHO cutoff of 20 ng/ml. Elevated levels have high specificity (98%). May be elevated after urticarial reactions; should not be elevated at baseline.
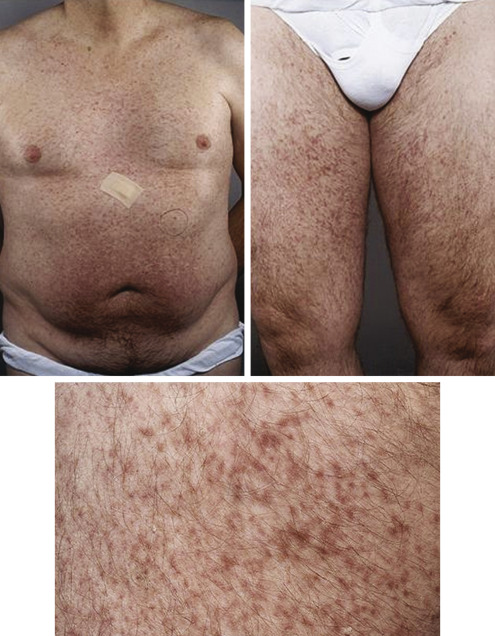
- Skin biopsy (Fig. E2) for urticarial pigmentosa/mastocytosis in the skin (MIS).
- Bone marrow examination (Fig. E3) in adults with MIS or elevated tryptase. In adult patients with cutaneous MC (mast cell) lesions, the large majority ultimately will be found to have SM according to WHO diagnostic criteria. A bone marrow biopsy usually is recommended in these patients to establish a diagnosis of SM (Fig. E4).
- Flow cytometry/immune histochemistry for aberrant CD25 and/or CD2 expression, in addition to normal mast cell markers.
- Molecular studies (polymerase chain reaction, PCR) for KIT D816V mutation, preferably on tissue (marrow/skin); peripheral blood studies are less sensitive. If KIT D816V is negative, consider evaluation for other KIT mutations.
- CBC and liver function studies; imaging of liver/spleen if appropriate.
- Bone density study for early osteoporosis in all patients; skeletal imaging if symptomatic (bone scan, plain x-rays).
- Different prognostic systems have been published to guide patient management in SM. In one of the more commonly used approaches, a large series of 1639 patients was used to develop the International Prognostic Scoring System for Mastocytosis (IPSM), which was then validated in 462 patients with SM.3 This four-category scoring system has been found to be useful in predicting survival outcomes and guiding treatment decisions.
TABLE E1 World Health Organization Diagnostic Criteria for Systemic Mastocytosis.a
Major Criterion
Multifocal, dense infiltrates of mast cells (>15 mast cells in aggregates) detected in sections of bone marrow and/or other extracutaneous organ(s) |
Minor Criteria- In biopsy sections of bone marrow or other extracutaneous organs, >25% of the mast cells in the infiltrate are spindle-shaped or have atypical morphology; or of all mast cells in bone marrow aspirate smears, >25% are immature or atypical.
- Detection of an activating point mutation at codon 816 in KIT in bone marrow, blood, or another extracutaneous organ.
- Mast cells in bone marrow, blood, or other extracutaneous organs express CD2 and/or CD25 in addition to normal mast cell markers.
- Serum total tryptase persistently exceeds 20 ng/ml (unless there is an associated clonal myeloid disorder, in which case this parameter is not valid).
|
From Hoffman R et al: Hematology: basic principles and practice, ed 7, Philadelphia, 2018, Elsevier.
TABLE E2 2016 World Health Organization Variants of Mastocytosis
- Cutaneous mastocytosis (CM)
- Maculopapular CM
- Diffuse CM
- Mastocytoma of skin
- Indolent systemic mastocytosis (ISM) a. Isolated bone marrow mastocytosis
- Smoldering systemic mastocytosis (SSM)
- Systemic mastocytosis with an associated hematologic neoplasm (SM-AHN)∗
- SM-MDS
- SM-MPN (e.g., PV, ET, MF, CML)
- SM-MDS/MPN (e.g., CMML, MDS/MPN-unclassified)
- SM-CEL
- SM-AML
- SM-lymphoid neoplasm (e.g., NHL, CLL, multiple myeloma)
- Aggressive systemic mastocytosis (ASM)
- Mast cell leukemia (MCL)
- Aleukemic MCL
- Mast cell sarcoma (MCS)
|
CLL, Chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; ET, essential thrombocythemia; MDS, myelodysplastic syndrome; MF, myelofibrosis; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; PV, polycythemia vera; SM-AML, systemic mastocytosis with acute myeloid leukemia; SM-CEL, systemic mastocytosis with chronic eosinophilic leukemia; SM-MDS, systemic mastocytosis with myelodysplastic syndrome; SM-MPN, systemic mastocytosis with myeloproliferative neoplasm.
From Hoffman R et al: Hematology: basic principles and practice, ed 7, Philadelphia, 2018, Elsevier.
TABLE E3 2016 World Health Organization Diagnostic Criteria for Variants of (Systemic) Mastocytosis
- Indolent systemic mastocytosis (ISM): Meets criteria for SM. No “B” or “C” findings. No evidence of associated clonal hematologic malignancy/disorder. In this variant, the mast cell burden is usually low; skin lesions are almost invariably present.
- Bone marrow mastocytosis: As above, with bone marrow involvement, but no skin lesions.
- Smoldering systemic mastocytosis (SSM): Meets criteria for SM. Two or more “B” findings and no “C” findings.
- Systemic mastocytosis with an associated hematologic neoplasm (SM-AHN)∗: Meets criteria for SM and an associated hematologic neoplasm (MDS, MPN, MDS/MPN, CEL, AML, lymphoma, or other hematologic neoplasm that meets the criteria for a distinct entity in the WHO classification).
- Aggressive systemic mastocytosis (ASM): Meets criteria for SM. One or more “C” findings. No associated clonal hematologic neoplasm. No evidence of mast cell leukemia.
- Mast cell leukemia (MCL): Meets criteria for SM. Bone marrow biopsy shows diffuse infiltration, usually in an interstitial pattern, by atypical, immature mast cells. Bone marrow aspirate smears show 20% or more mast cells. Cases in which <10% of circulating WBCs are mast cells are referred to as “aleukemic mast cell leukemia.”
- Mast cell sarcoma (MCS): Unifocal mast cell tumor. No evidence of SM. No skin lesions. Destructive growth pattern. High-grade cytology.
|
AML, Acute myeloid leukemia; CEL, chronic eosinophilic leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; SM, systemic mastocytosis; WBC, white blood cell; WHO, World Health Organization.
From Hoffman R et al: Hematology: basic principles and practice, ed 7, Philadelphia, 2018, Elsevier.
TABLE E4 “B” Findings: Indication of High Mast Cell Burden
- Infiltration grade (mast cells) greater than 30% in bone marrow in histology and serum total tryptase levels greater than 200 ng/ml
- Hypercellular marrow with loss of fat cells, discrete signs of dysmyelopoiesis without substantial cytopenias, and without WHO criteria for an MDS or MPN
- Organomegaly: Palpable hepatomegaly, splenomegaly, or lymphadenopathy (on CT or ultrasound) greater than 2 cm without impaired organ function
|
CT, Computed tomography; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; WHO, World Health Organization.
Figure E2 Cutaneous mastocytosis.
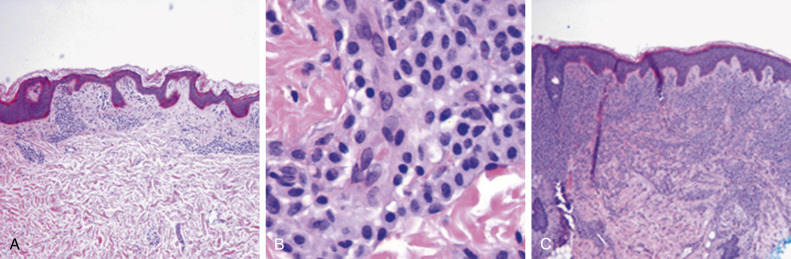
A skin biopsy of urticaria pigmentosa or maculopapular mastocytosis shows multiple focal aggregates of mast cells around blood vessels or skin appendages in the papillary dermis (A). The mast cells are plump with abundant cytoplasm and typically accumulate around vessels (B, center). In solitary mastocytoma of the skin or in diffuse cutaneous mastocytosis, the mast cell infiltrate is more extensive as it infiltrates the papillary and reticular dermis and even may extend into the subcutaneous tissues (C). The mast cells are bland and without cytologic atypia. In these disorders, there should be no evidence of systemic involvement. Evidence of systemic disease would indicate systemic mastocytosis.
From Hoffman R [ed]: Hematology: basic principles and practice, ed 6, Philadelphia, 2013, Elsevier/Churchill Livingstone.
Figure E3 Well-differentiated systemic mastocytosis.
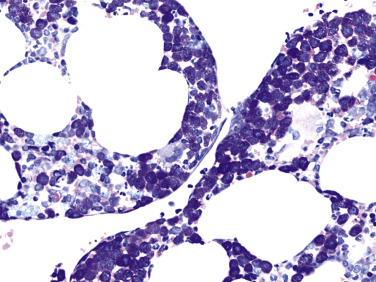
Core biopsy, Giemsa stain. Bone marrow shows aggregates of exclusively round mast cells with an abundance of metachromatic granules. This is the typical phenotypical appearance of a well-differentiated systemic mastocytosis. The disease otherwise was subtyped as indolent systemic mastocytosis with skin involvement but missing the KIT D816V mutation. There also was a lack of CD25 expression by the mast cells (not depicted).
From Hoffman R et al: Hematology: basic principles and practice, ed 7, Philadelphia, 2018, Elsevier.
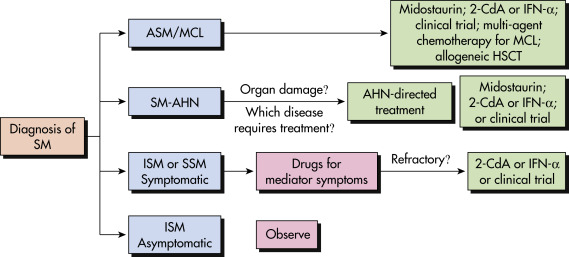
Figure E4 Diagnostic Decision Pathways for Patients with Mast Cell Activation Symptoms or Adult-Onset Mastocytosis in the Skin
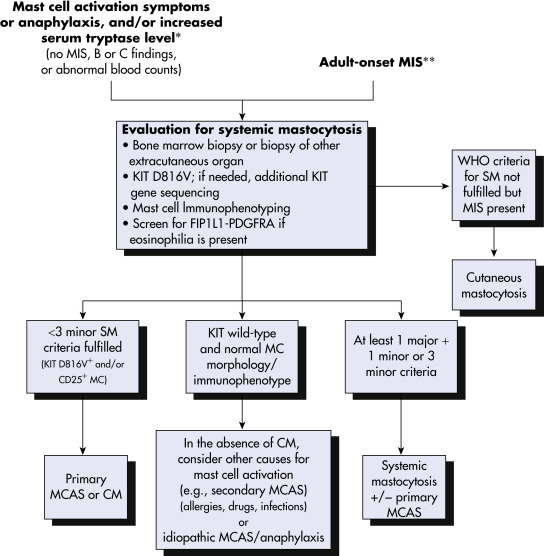
For patients with mast cell activation symptoms or anaphylaxis (and/or increased serum tryptase level), or adult-onset MIS, screening to assess whether diagnostic criteria for SM are met should be the first diagnostic checkpoint. In patients not meeting the criteria for SM, evaluation for MCAS (primary vs. secondary vs. idiopathic) and idiopathic anaphylaxis is the next phase. Patients with MIS in whom no signs of SM can be found may be categorized as having cutaneous mastocytosis. ∗The serum tryptase level may be below the 20 ng/ml threshold, or only transiently elevated. ∗∗Although an evaluation for systemic mastocytosis generally is recommended in adults with MIS who have no blood count abnormalities or organ dysfunction and a normal or mildly increased serum tryptase level, the value of performing a bone marrow biopsy should be discussed with the patient. CM, Cutaneous mastocytosis; MC, mast cell; MCAS, mast cell activation syndrome; MIS, mastocytosis in the skin; SM, systemic mastocytosis; PDGFRA, platelet-derived growth factor receptor A; WHO, World Health Organization.
Adapted from Pardanani A: How I treat patients with indolent and smoldering mastocytosis [rare conditions but difficult to manage], Blood 121:3085, 2013; Valent P et al: Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus protocol, Int Arch Allergy Immunol 157:215, 2012. In Hoffman R et al: Hematology: basic principles and practice, ed 7, Philadelphia, 2018, Elsevier.