AUTHOR: Robert H. Janigian Jr., MD
Diabetic retinopathy (Fig. 1) is a microvascular abnormality of the retina resulting in microaneurysms (Fig. E2), retinal hemorrhages, lipid exudates (Fig. E3), macular edema, and neovascular vessel growth (Fig. E4) and can ultimately end in blindness. Diabetic retinopathy can be classified into two stages: Nonproliferative (mild, moderate, and severe) and proliferative. A glossary and abbreviations pertinent to diabetic eye disease are described in Table E1.
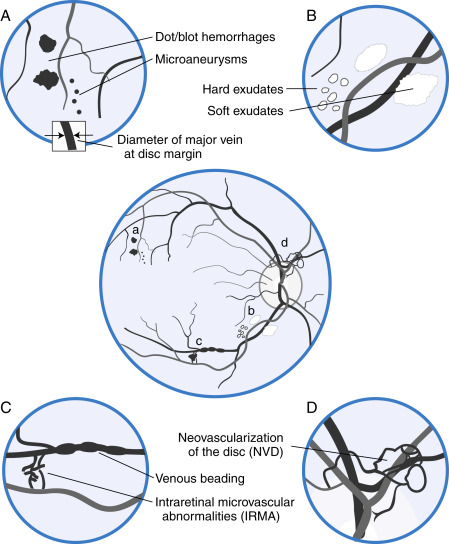
Figure 1 Types of diabetic retinopathy.
The center figure depicting the fundus of a patient with diabetic retinopathy is surrounded by four enlarged views, each labeled with a letter (a to d) corresponding to specific locations on the center figure. A, Microaneurysms and dot and blot hemorrhages. The diameter of microaneurysms is less than the width of a major vein at the disc margin (reproduced in square inset). B, Hard and soft exudates. C, Venous beading and intraretinal microvascular abnormalities (IRMA). D, Neovascularization, which may be located within one disc diameter of the optic disc (NVD) or elsewhere (NVE). Although both IRMA and neovascularization represent the formation of new blood vessels, IRMA are confined to the layers of the retina, whereas neovascularization is on the inner surface of the retina or vitreous.
From McGee S: Evidence-based physical diagnosis, ed 4, Philadelphia, 2018, Elsevier.
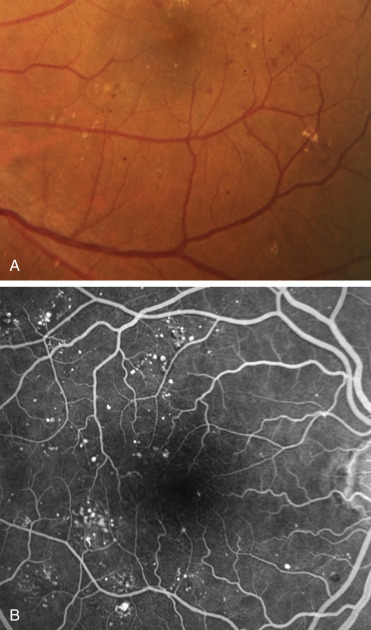
A, Microaneurysms and dot/blot hemorrhages at the posterior pole. B, Fluorescein angiogram (FA) shows scattered hyperfluorescent spots consistent with microaneurysms in the posterior fundus.
From Bowling B: Kanski’s clinical ophthalmology, a systematic approach, ed 8, Philadelphia, 2016, Elsevier.
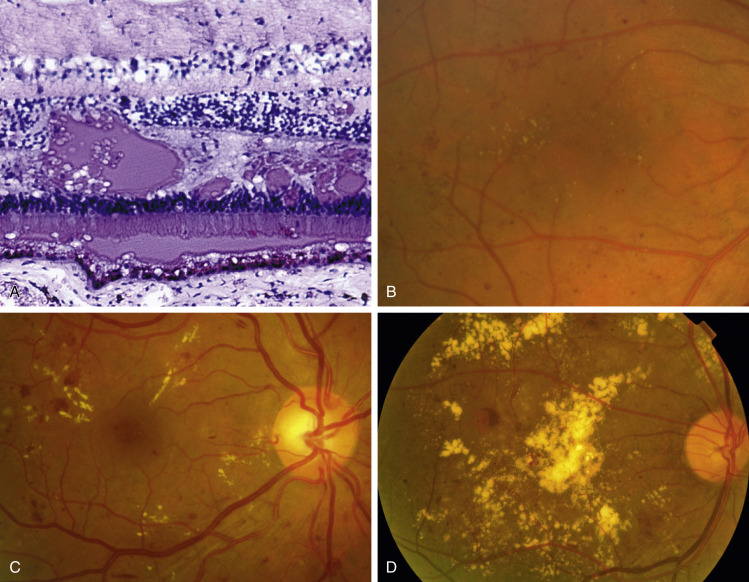
A, Histology shows irregular eosinophilic deposits mainly in the outer plexiform layer. B, Small exudates and microaneurysms. C, More extensive exudates, some associated with microaneurysms. D, Exudates involving the fovea, including central crystalline lipoprotein deposition-focal laser has recently been applied superotemporal to the fovea.
Courtesy J. Harry J [A] and S. Chen [C and D]. In Bowling B: Kanski’s clinical ophthalmology, a systematic approach, ed 8, Philadelphia, 2016, Elsevier.
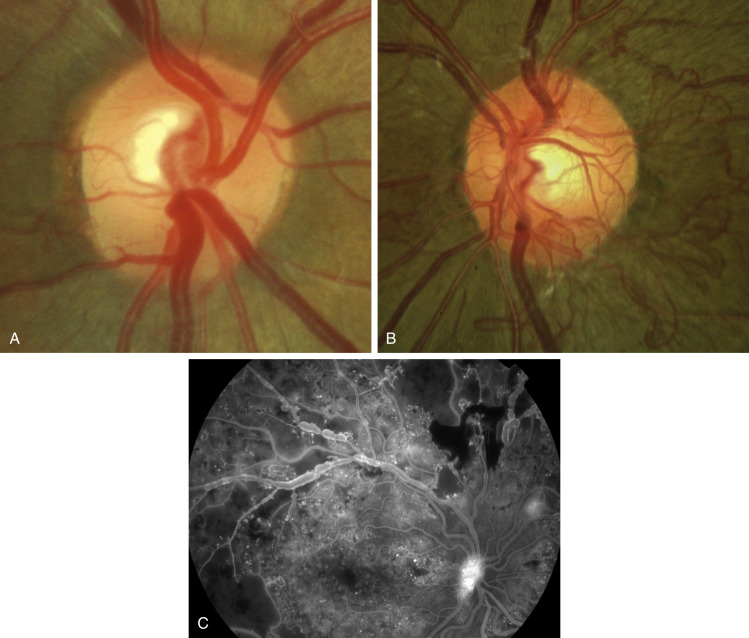
A, Mild. B, Severe. C, Fluorescein angiogram (FA) shows leaking disc vessels, with extensive peripheral capillary dropout and a small focus of leaking vessels elsewhere.
Courtesy S. Chen [B]. In Bowling B: Kanski’s clinical ophthalmology, a systematic approach, ed 8, Philadelphia, 2016, Elsevier.
TABLE E1 Glossary and Abbreviations Pertinent to Diabetic Eye Disease
| Term | Definition | ||
|---|---|---|---|
| Antivascular endothelial growth factor (anti-VEGF) therapies; VEGF inhibitors | For the purposes of this chapter, these refer to VEGF inhibitors (including aflibercept, bevacizumab, and ranibizumab) that are given intravitreally for the treatment of diabetic macular edema and proliferative diabetic retinopathy. | ||
| Background diabetic retinopathy (BDR) | An outdated term referring to some stages of NPDR; not closely associated with disease progression; replaced by the various levels of NPDR. | ||
| Center-involved (or central-involved) diabetic macular edema (ciDME) | Abnormal thickening of the central retina (usually 1 mm in diameter central retinal subfield) due to increased vascular permeability. Because central involvement is more likely to cause visual impairment, it is commonly used as a threshold for treatment. | ||
| Clinically significant macular edema (CSME) | Thickening of the retina in the macular region of sufficient extent and location to threaten central visual function. | ||
| Cotton-wool spot | A gray or white area lesion in the nerve fiber layer of the retina resulting from stasis of axoplasmic flow caused by microinfarcts of the retinal nerve fiber layer. | ||
| Diabetes Control and Complications Trial (DCCT) | A multicenter, randomized clinical trial designed to address whether intensive insulin therapy could prevent or slow the progression of systemic complications of diabetes mellitus. | ||
| Diabetic retinopathy (DR) | Retinal damage related to the underlying systemic disease of diabetes mellitus. | ||
| Diabetic Retinopathy Study (DRS) | The first multicenter, randomized clinical trial to demonstrate the value of scatter (panretinal) photocoagulation in reducing the risk of vision loss among patients with all levels of diabetic retinopathy. | ||
| Diabetic Retinopathy Vitrectomy Study (DRVS) | A multicenter clinical trial evaluating early vitrectomy for patients with very advanced diabetic retinopathy or nonresolving vitreous hemorrhage. | ||
| Early Treatment Diabetic Retinopathy Study (ETDRS) | A multicenter, randomized clinical trial that addressed at what stage of retinopathy scatter (panretinal) photocoagulation was indicated, whether focal photocoagulation was effective for preventing moderate vision loss due to clinically significant macular edema, and whether aspirin therapy altered the progression of diabetic retinopathy. | ||
| Focal or grid laser photocoagulation | A type of laser treatment whose main goal is to reduce vascular leakage, either by focal treatment of leaking retinal microaneurysms or by application of therapy in a gridlike pattern for patients with clinically significant macular edema. | ||
| Hard exudate | Lipid accumulation within the retina as a result of increased vasopermeability. | ||
| High-risk characteristic proliferative diabetic retinopathy (HRC-PDR) | Proliferative diabetic retinopathy of defined extent, location, or clinical findings that is particularly associated with severe vision loss. | ||
| Microaneurysm | An early vascular abnormality consisting of an outpouching of the retinal microvasculature. | ||
| Neovascular glaucoma (NVG) | Elevation of intraocular pressure caused by the development of neovascularization in the anterior segment of the eye. | ||
| Neovascularization at the disc (NVD) | Retinal neovascularization occurring at or within 1500 μm of the optic disc. | ||
| Neovascularization elsewhere (NVE) | Retinal neovascularization that is located more than 1500 μm away from the optic disc. | ||
| Neovascularization of the iris (NVI) | Neovascularization occurring on the iris (rubeosis iridis), usually as a result of extensive retinal ischemia. | ||
| No light perception (NLP) | The inability to perceive light. | ||
| Nonproliferative diabetic retinopathy (NPDR) | Severities (mild, moderate, severe) of clinically evident diabetic retinopathy that precede the development of PDR. | ||
| Preproliferative diabetic retinopathy (PPDR) | An outdated term referring to more advanced levels of NPDR; not closely associated with disease progression; replaced by the various levels of NPDR. | ||
| Proliferative diabetic retinopathy (PDR) | An advanced level of diabetic retinopathy in which proliferation of new vessels or fibrous tissue occurs on or within the retina. | ||
| Rubeosis iridis | Neovascularization of the iris. |
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.
Nonproliferative diabetic retinopathy (NPDR)
Proliferative diabetic retinopathy (PDR)
| ||||||||||||||||||||||||||||||||||||||||||||
- After 20 yr of diabetes mellitus, nearly 99% of patients with type 1 and 60% with type 2 disease demonstrate some degree of diabetic retinopathy.
- A leading preventable cause of blindness in the U.S. between the ages of 20 and 74 yr.
- There are approximately 8000 new cases of diabetic retinopathy-induced blindness each year in the U.S.
- Prevalence of retinopathy increases with duration of diabetes. Found in 18% of people diagnosed with diabetes for 3- to 4-yr duration and in up to 80% of diabetics with a diagnosis of ≥15 yr.
- In the U.S., the prevalence of diabetic macular edema (the most common cause of vision impairment) is 4%, or approximately 750,000 people.
- Prevalence of diabetic retinopathy in patients 40 and older is 28.5%, or approximately 4.4 million people.
- The number of Americans with diabetic retinopathy is expected to nearly double, from 7.7 million to over 13 million between 2010 and 2050. Expected to triple in the Hispanic population.
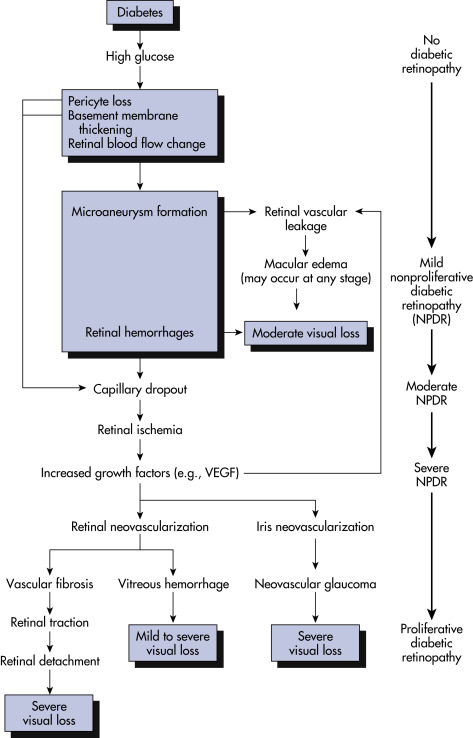
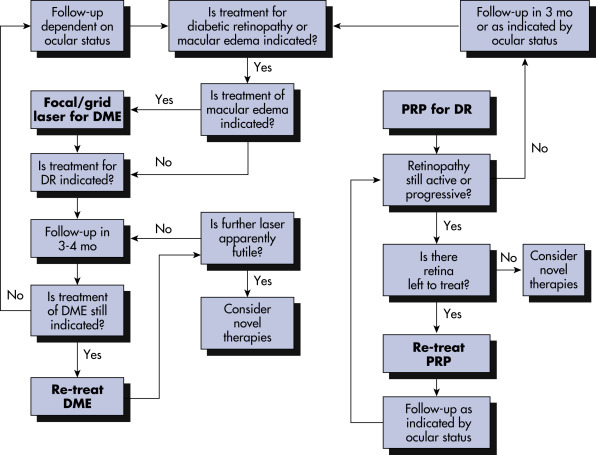
Prolonged hyperglycemia results in basement membrane thickening in retinal capillaries with subsequent loss of pericytes and endothelial decompensation resulting in breakdown of the blood-retina barrier and capillary occlusion, ischemia, and serum leakage (Fig. 5). This leads to the clinical findings of dot hemorrhages, microaneurysms, lipid exudate, and edema. Upregulation of vascular endothelial growth factor (VEGF) and other cytokines contributes to vascular incompetence and promotes growth of neovascular tissue.
Figure 5 Pathogenesis of diabetic retinopathy.

This schematic flow chart represents the major preclinical and clinical findings associated with the full spectrum of diabetic retinopathy and macular edema. VEGF, Vascular endothelial growth factor.
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.