AUTHORS: Frank B. D’ Alessandro, MD, and Fred F. Ferri, MD
- Diabetes mellitus (DM) refers to a syndrome of hyperglycemia resulting from many different causes (see “Etiology”). It is broadly classified into type 1 (T1DM) and type 2 DM (T2DM). The terms insulin-dependent and non-insulin-dependent diabetes are obsolete because when a person with type 2 diabetes needs insulin, he or she remains labeled as type 2 and is not reclassified as type 1. Immune-mediated type 1 DM (type 1A) represents 5% to 10% of newly diagnosed diabetics. Tables 1 and 2 provide a general comparison of the two types of DM. One difference is that type 1 has usually complete or near-total knockout of insulin reserves mediated solely by immunogenic responses from carriers of certain genotypes, whereas type 2 is of polygenetic origin and may have patients who may start with hyperinsulinemia but have insulin resistance and through environmental factors such as diet and sedentary lifestyle leads to an imbalance between glucagon and insulin levels, resulting in combination of causes toward hyperglycemia.
- Some type 1 diabetics also may exhibit high levels of glucagon, and not all type 1 diabetics have complete islet cell destruction.
- The classification of diabetes also includes:
- LADA: Latent autoimmune diabetes of adult onset (sometimes called type 1.5 DM). These individuals are typically not insulin dependent initially and are often misclassified as having type 2 DM.
- MODY: Maturity onset diabetes of youth. These have various genetic expressions and can be classified into various subtypes:
- Ketosis-prone diabetes: Relapsing/remitting beta cell function with slow deterioration over time. It presents with ketoacidosis requiring insulin, then regains beta cell function, and patient is able to discontinue insulin. This for is most common under age 40, in those of African or Afro-Caribbean origin, and in obese or overweight patients.
- Secondary diabetes:
- Rare autoimmune (e.g., type A and B insulin resistance syndrome).
- A classification of diabetes mellitus is shown in Box 1.
- Diabetes mellitus can be diagnosed by the following tests:
- A hemoglobin A1c (HbA1c) value ≥6.5% is considered diagnostic for diabetes. This test is preferred because of ease of administration and reliability.
- A fasting plasma glucose (FPG) ≥126 mg/dl, which should be confirmed with repeat testing on a different day. Fasting is defined as no caloric intake for at least 8 h.
- An oral glucose tolerance test (OGTT) with a plasma glucose ≥200 mg/dl 2 h after a 75 g (100 g for pregnant women) glucose load.
- Symptoms of hyperglycemia and a casual (random) plasma glucose ≥200 mg/dl are also indicative of DM. Classic symptoms of hyperglycemia include polyuria, polydipsia, and unexplained weight loss. At the time of diagnosis as a diabetic, B-cell function is at 25% to 30%.
- Individuals with glucose levels higher than normal but not high enough to meet the criteria for diagnosis of DM are considered to have “prediabetes,” the diagnosis of which is made as follows:
- A fasting plasma glucose 100 to 125 mg/dl; this is referred to as impaired fasting glucose.
- After OGTT, a 2-h plasma glucose of 140 to 199; this is referred to as impaired glucose tolerance. Patients with impaired glucose tolerance or prediabetes have B-cell function at 50% of normal.
- A hemoglobin A1c value of 5.7% to 6.4%.
- Table 3 describes diagnostic categories for DM and at-risk states.
TABLE 3 Diagnostic Categories∗: Diabetes Mellitus and At-Risk States
| Fasting Plasma Glucose Level | 2-hr (75-g) OGTT Result | ||
|---|---|---|---|
| <140 mg/dl | 140-199 mg/dl | ≥200 mg/dl | |
| <100 mg/dl | Normal | IGT† | DM |
| 100-125 mg/dl | IFG† | IGT† and IFG† | DM |
| ≥126 mg/dl | DM | DM | DM |
| HbA1CLevel | <5.7% | 5.7-6.4% | ≥6.5% |
| Normal | High-risk† | DM | |
DM, Diabetes mellitus; HbA1c, glycosylated hemoglobin; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test.
∗These diagnostic categories are based on the combined fasting plasma glucose level and a 2-h, 75-g oral glucose tolerance test (OGTT) result. Note that a confirmed random plasma glucose level of 200 mg/dl or higher in the appropriate clinical setting is diagnostic of diabetes and precludes the need for further testing.
† May be referred to as prediabetes.
From Goldman L, Schafer AI: Goldman’s Cecil medicine, ed 24, Philadelphia, 2012, Saunders.
Box 1 Classification of Diabetes Mellitus
From McPherson RA, Pincus MR: Henry’s clinical diagnosis and management by laboratory method, ed 23, St. Louis, 2017, Elsevier.
TABLE 1 Characteristic Comparison of Type 1 Versus Type 2 Diabetes Mellitus
| Characteristic | Type 1 | Type 2 |
|---|---|---|
| Nature Very different | Autoimmune disorder marked by destruction of insulin-producing beta cells and loss of insulin production | A disorder of insulin deficiency involving an interplay between both pancreatic and extrapancreatic contributions to disease |
| Symptoms Partial overlap | Rapid onset; very high to extremely high blood glucose levels; polyphagia; polydipsia, polyuria; ketoacidosis | Mild to moderate onset; modest to high elevations in blood glucose; mild polydipsia/polyuria; fatigue; visual changes/headache |
| Onset Very different | Sudden (symptoms for days to weeks) | Slower onset (symptoms for mos to yrs) |
| Risk factors Typically different but overlap | Family history of autoimmune disease but particularly type 1 diabetes mellitus (10-fold increased risk vs. general population) | Overweight/obese; poor diet; sedentary lifestyle; ethnicity (higher in African Americans, Hispanics); family history of type 2 diabetes mellitus; history of gestational diabetes |
| Onset age Typically different but overlap | Typically early life through adolescence but can occur at any age | Typically adults but trending toward earlier age of onset |
| Treatment strategy Typically different | Absolute requirement for insulin (multiple daily injections or insulin pump); self-management lifestyle modification (monitor food types, exercise, etc.) | Dietary modifications and exercise alongside oral agents (for most); increasingly greater percentage of patients require insulin over time |
| Can it be prevented? Very different | Not at present (subject of major research efforts); future cases can be predicted by autoantibodies and genetics | Yes, for more than half of potential cases, with dietary modifications and exercise |
| Can it be reversed? Very different | Not at present (subject of major research efforts) | No, but for a limited few; patients can see disease managed and risk for complications reduced through diet modifications, exercise; growing evidence for disease improvements through combination therapies |
| Complications Mostly similar, but some variation | Acute emergencies of hypoglycemia and ketoacidosis leading to hypoglycemic unawareness; chronic effects of hyperglycemia can lead to retinopathy, nephropathy, neuropathy, cardiovascular disease, etc. | Acute emergencies of hypoglycemia and ketoacidosis leading to hypoglycemic unawareness; chronic effects of hyperglycemia can lead to retinopathy, nephropathy, neuropathy, cardiovascular disease, etc. |
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.
TABLE 2 Characteristics of Type 1 and Type 2 Diabetes Mellitus
| Type 1 Diabetes | Type 2 Diabetes | |
|---|---|---|
| Frequency | 5%-10% | 90%-95% |
| Age of onset | Any, but most common in children and young adults | More common with advancing age, but can occur in children and adolescents |
| Risk factors | Genetic, autoimmune, environmental | Genetic, obesity, sedentary lifestyle, race/ethnicity, hypertension, dyslipidemia, polycystic ovarian syndrome |
| Pathogenesis | Destruction of pancreatic beta cells, usually autoimmune | No autoimmunity Insulin resistance and progressive insulin deficiency |
| C-peptide levels | Very low or undetectable | Detectable |
| Prediabetes | Autoantibodies (GAD65, IA-2, IAA, ZnT8) may be present | Autoantibodies absent |
| Medication therapy | Insulin absolutely necessary; multiple daily injections or insulin pump | Oral agents and/or noninsulin injectable hypoglycemic drugs Insulin commonly needed |
| Therapy to prevent or delay onset of diabetes | Teplizumab | Lifestyle (weight loss and increased physical activity) Oral medications (metformin, acarbose) may be helpful. |
Modified from McPherson RA, Pincus MR: Henry’s clinical diagnosis and management by laboratory method, ed 23, St. Louis, 2017, Elsevier.
IDDM (insulin-dependent diabetes mellitus)
NIDDM (non-insulin-dependent diabetes mellitus)
Type 1 diabetes mellitus (insulin-dependent diabetes mellitus)
Type 2 diabetes mellitus (non-insulin-dependent diabetes mellitus)
LADA (latent autoimmune diabetes of adult)
MODY (mature onset diabetes of youth)
| ||||||||||||||||||||||||||||||||||||||||||||
- DM affects 9% to 10% of the U.S. population. Prevalence rates vary considerably by race/ethnicity. T1DM accounts for approximately 5% of diagnosed diabetes cases and is defined by the presence of one or more autoimmune markers.
- In the U.S., the overall pediatric incidence of T1DM is approximately 25 per 100,000 per year. Incidence a varies according to age (peaking in the pubertal years, ages 10 to 14), season (winter more than summer), geographic location (Finland and other Nordic countries more than equatorial regions), and race and ethnic group (U.S. non-Hispanic White persons more than Native Americans).1
- Risk factors for type 1 DM are summarized in Table 4.
- There are three stages of type 1 DM. Patients in stage 1 have two or more diabetes-related autoantibodies, and those in stage 2 have developed asymptomatic dysglycemia but have normal other metabolic indices and do not require insulin treatment. Stage 3 is symptomatic clinical disease. Teplizumab is an anti-CD3 monoclonal antibody recently FDA approved to delay the onset of stage 3 type 1 DM in patients ≥8 years old who have stage 2 type 1 DM.1a
- While incidence of diabetes in adolescents is mostly type 1, the rate of type 2 being diagnosed in adolescents has increased by 1.5 times in certain given areas. This seems to correlate with the epidemic of pediatric obesity.
- Table 5 summarizes epidemiologic determinants of and risk factors for type 2 DM.
- Incidence rate increases with age, varying from 2% in persons age 20 to 44 yr to 18% in persons 65 to 74 yr. T2DM can have a long presymptomatic phase, leading to a 4- to 7-yr delay in diagnosis. In the U.S. 1.2 million new cases of diabetes are diagnosed each yr, and 86 million have prediabetes. Currently, 30 million Americans have diabetes; with this current trend, it is predicted that by 2050, 1 out of 3 Americans will be diabetic.
- Diabetes accounts for 8% of all legal blindness in the U.S. and is the leading cause of end-stage renal disease (ESRD). Approximately 40% of patients in a given dialysis center are diabetic.
- Patients with diabetes are two to four times more likely than nondiabetic patients to experience development of cardiovascular disease (CVD).
TABLE 5 Epidemiologic Determinants of and Risk Factors for Type 2 Diabetes Mellitus
| Genetic Factors | |||
| Genetic markers Family history | |||
| Demographic Characteristics | |||
| Age Ethnicity | |||
| Behavioral and Lifestyle-Related Risk Factors | |||
| Obesity (including distribution of obesity and duration) Physical inactivity Diet Stress Westernization, urbanization, modernization Medications Shift work | |||
| Metabolic Determinants and Intermediate-Risk Categories of Type 2 Diabetes | |||
| Impaired glucose tolerance Insulin resistance Gestational diabetes Offspring of women with diabetes during pregnancy Intrauterine malnutrition or overnutrition Microbiome composition |
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.
TABLE 4 Risk of Type 1 Diabetes Mellitus
| Group | Childhood Annual Incidence | ||
|---|---|---|---|
| U.S. general population | 0.3% (15-25/100,000) | ||
| Offspring | 1% | ||
| Sibling | 3.2% (through adolescence); 6% lifetime | ||
| Dizygotic twin | 6% | ||
| Mother | 2% | ||
| Father | 4.6% | ||
| Both parents | ∼10% | ||
| Monozygotic twin | 50%, but incidence varies with age of index twin |
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.
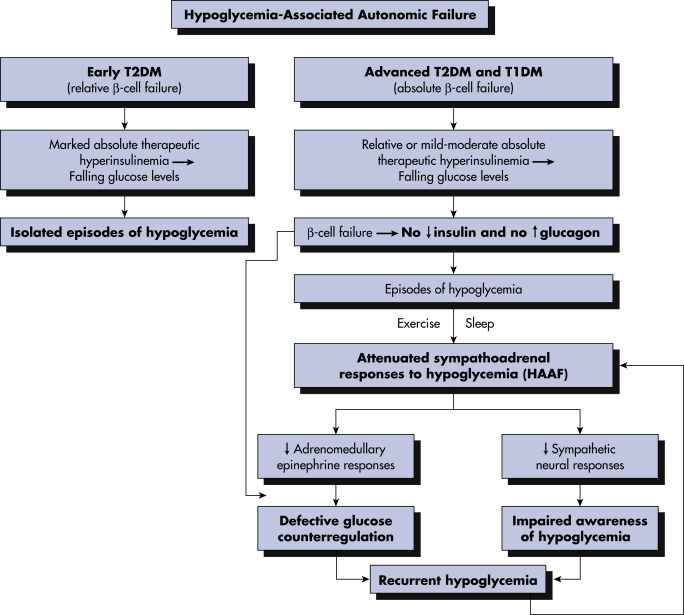
- Physical examination varies with the presence of complications and may be normal in early stages
- Diabetic retinopathy:
- Nonproliferative (background diabetic retinopathy):
- Initially: Microaneurysms, capillary dilation, waxy or hard exudates, dot and flame hemorrhages, arteriovenous shunts
- Advanced stage: Microinfarcts with cotton wool exudates, macular edema
- Proliferative retinopathy: Characterized by formation of new vessels, vitreous hemorrhages, fibrous scarring, and retinal detachment
- Nonproliferative (background diabetic retinopathy):
- Cataracts and glaucoma occur with increased frequency in patients with diabetes
- Diabetic macular edema: Swelling of the macula leading to loss of sharp vision in this part of the eye. People with this disorder usually already have retinopathy but may present separately as well
- Diabetic neuropathy:
- Distal sensorimotor polyneuropathy:
- Symptoms include paresthesia, hyperesthesia, or burning pain involving bilateral distal extremities in a “stocking glove” distribution. This can progress to motor weakness and ataxia
- Physical examination may reveal decreased pinprick sensation, decreased sensation to light touch, decreased vibration sense, and loss of proprioception. Motor disturbances such as decreased deep tendon reflexes and atrophy of interosseous muscles can also be seen
- Autonomic neuropathy:
- GI disturbances: Esophageal motility abnormalities, gastroparesis, diarrhea (usually nocturnal):
- Genitourinary (GU) disturbances: Neurogenic bladder (hesitancy, weak stream, and dribbling), impotence
- Cardiovascular (CV) disturbances: Orthostatic hypotension, tachycardia, decreased heart rate variability (HRV). Decreased HRV is associated with increased cardiac mortality, independent of ejection fraction
- Polyradiculopathy: Painful weakness and atrophy in the distribution of ≥1 contiguous nerve root
- Mononeuropathy involving cranial nerves III, IV, or VI or peripheral nerves can also occur
- Distal sensorimotor polyneuropathy:
- Diabetic nephropathy: Pedal edema, pallor, weakness, uremic appearance
- Nephrotic syndrome: Proteinuria, hypertriglyceridemia, edema
- Nephritis: Progressive degeneration of nephrons focal glomerulosclerosis
Type IV renal tubular acidosis, hyperkalemic nephropathy, interstitial nephritis, causing hyporeninemic hypoaldosteronism. It is important to keep in mind that NSAIDs, ACE inhibitors, trimethoprim, and heparin can all reduce aldosterone and can cause or exacerbate the condition. 50% of patients on dialysis have diabetes as primary diagnosis; whereas glycemia is important to prevent nephropathy, the most significant contributing factor is hypertension.
- Foot ulcers: Occur in 15% of individuals with diabetes (annual incidence rate 2%) and are the leading causes of hospitalization; they are usually secondary to a combination of factors, including peripheral vascular insufficiency, repeated trauma (unrecognized because of sensory loss), and superimposed infection.
- Patient symptoms are usually less than would be expected from clinical findings, due to loss of sensation related to peripheral neuropathy.
- Comprehensive foot exams include visual inspection, assessment of pedal pulses, and assessment of protective sensation using a 10-g monofilament to test sensation.
- Prevention of foot ulcers in an individual with diabetes includes strict glucose control, patient education, prescription footwear, intensive podiatric care, and evaluation for surgical interventions.
- Foot examination should be done annually on all diabetics.
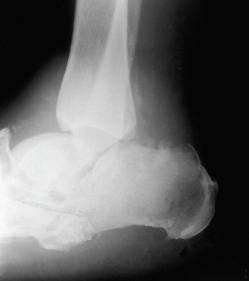
- Neuropathic arthropathy (Charcot joints): Bone or joint deformities from repeated trauma (secondary to peripheral neuropathy; Fig. E1).
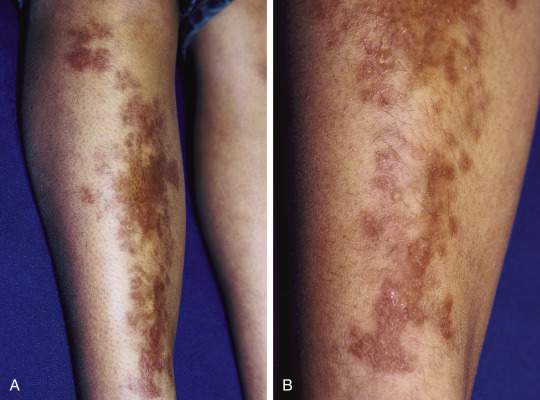
- Necrobiosis lipoidica diabeticorum: Plaque-like reddened areas with a central area that fades to white-yellow, found on the anterior surfaces of the legs (Fig. E2); in these areas, the skin becomes very thin and can ulcerate easily.
- Other diabetic skin manifestations with diabetes include:
- Scleroderma diabeticorum: Thickening of skin and epidermis giving skin a “leather-like texture”; typically affects type 2, mainly on upper back and neck.
- Dermatitis herpetiformis: Diffuse petechial rash associated with gluten insensitivity.
- Vitiligo: Associated with type 1 and type 2 and affects skin coloration due to autoimmune reaction to pigmentation (at times may be associated with adrenal insufficiency, especially in type 1 patients). Patients should use SPF 30 sunscreen to prevent sunburn.
- Acanthosis nigricans: Darkening of skin folds in neck and axilla, at times raised and velvety, thought to be associated with insulin resistance; other conditions such as Cushing and acromegaly can have this as well.
- Diabetic dermopathy: Appears as shiny round or oval lesions on thin skin of the lower extremity, also known as “shin spots”; these are usually not painful and do not require treatment.
- Eruptive xanthomatosis: Associated with uncontrolled blood sugars and extremely high triglycerides. There is a high risk for pancreatitis in patients with this finding; treatment is aimed at lowering blood sugars with insulin and fibrates.
- Digital sclerosis: Skin on toes fingers and hands become waxy, thick, and tight, with joint stiffness; treatment is aimed at lowering blood sugars and encouraging use of lotions and moisturizers.
- Bullosis diabeticorum: Rare disorder manifesting with blisters on hands, forearms, toes, and feet, usually painless. Lesions generally heal on their own.
- Box 2 summarizes cutaneous associations with diabetes mellitus.
Box 2 Some Cutaneous Associations With Diabetes Mellitus
From Paller AS, Mancini AJ: Hurwitz clinical pediatric dermatology: a textbook of skin disorders of childhood and adolescence, ed 5, Philadelphia, 2016, Elsevier.
Figure E1 Diabetic neuropathy of the hindfoot.
Destruction of the joint with collapse and fragmentation.
From Hochberg MC et al [eds]: Rheumatology, ed 3, St Louis, 2003, Mosby.
Figure E2 Necrobiosis lipoidica diabeticorum.
Waxy, yellow-brown plaques (A) were present in this young girl with diabetes mellitus, with subtle atrophy present on closer inspection (B).
From Paller AS, Mancini AJ: Hurwitz clinical pediatric dermatology, a textbook of skin disorders of childhood and adolescence, ed 5, Philadelphia, 2016, Elsevier.
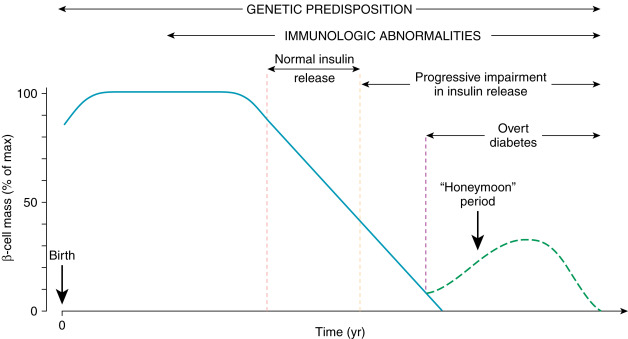
Type 1 DM: Results from autoimmune β-cell destruction, usually leading to absolute insulin deficiency (Fig. 3).
From Marcdante KJ et al: Nelson Essentials of Pediatrics, ed 9, Philadelphia, 2023, Elsevier.
Type 2 DM: Results from insulin resistance and a progressive defect in insulin secretion.
- Hormonal excess: Cushing syndrome, acromegaly, glucagonoma, pheochromocytoma
- Drugs: Glucocorticoids, diuretics, oral contraceptives
- Insulin receptor unavailability (with or without circulating antibodies)
- Pancreatic disease: Pancreatitis, pancreatectomy, hemochromatosis, cystic fibrosis
- Genetic syndromes: Maturity onset diabetes of the young (MODY, monogenetic diabetes accounting for 2% to 5% of diabetes), familial hyperlipidemias, myotonic dystrophy, lipoatrophy
- Gestational diabetes (GDM): Diabetes diagnosed during pregnancy that is due to pregnancy-related insulin resistance