AUTHOR: Jessica E. Shill, MD
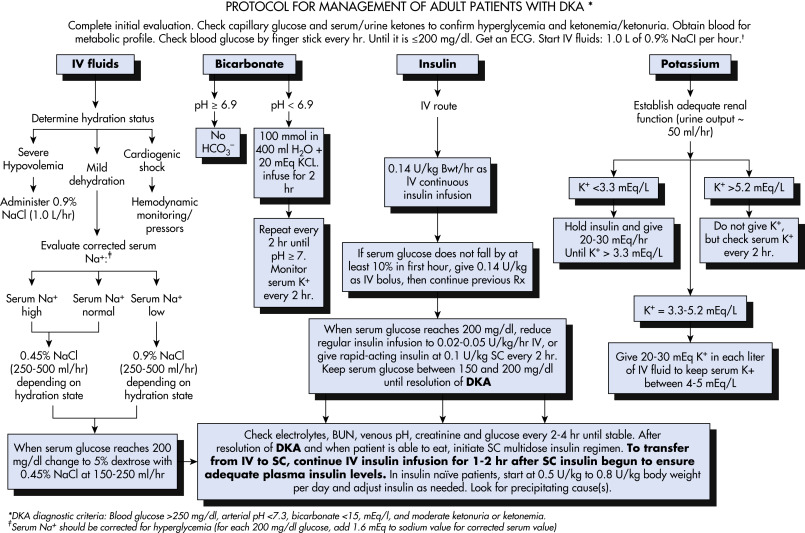
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes mellitus. It results from an absolute or relative insulin deficiency that results in insulin resistance when paired with counterregulatory hormone and free fatty acid excess. DKA is characterized by the presence of an anion gap metabolic acidosis, ketonemia, and hyperglycemia.
| ||||||||||||||||||||||||||||
DKA is the most common hyperglycemic emergency among patients with type 1 (T1D) and type 2 diabetes (T2D). In the past decade, the frequency of DKA has increased in the U.S., with more than 160,000 hospital admissions in 2017.1 Social and racial-ethnic disparities are remarkable, with Black race/ethnicity and lower income individuals at heightened risk of DKA.2 DKA most commonly occurs in individuals with T1D, with about one third of cases occurring in those with T2D. Those with ketosis-prone T2D are especially vulnerable. Overall, prevalence of DKA has increased, yet mortality has decreased to <5%, which is significantly lower than mortality from hyperglycemic hyperosmolar syndrome. Mortality from DKA in children and adolescents is most commonly due to cerebral edema, whereas in adults it is usually related to the precipitating illness (e.g., sepsis, cardiac or central nervous system ischemia, pneumonia). Older adult patients may present with multiple comorbidities that can complicate DKA even further.3 The most common precipitating factor for DKA in older adults is related to insulin therapy nonadherence and underlying comorbidities. These patients often present with sepsis and, frequently, with atrial fibrillation. The combination of diabetes with atrial fibrillation increases morbidity and mortality associated with atrial fibrillation. In addition, older adult patients may be prescribed antipsychotic medications for underlying dementia, and this situation has been associated with a higher incidence of DKA admissions.
- Polyuria, polydipsia, polyphagia, weight loss, weakness
- Signs of dehydration (tachycardia, hypotension, dry mucous membranes, sunken eyes, poor skin turgor)
- Nausea, vomiting, abdominal tenderness, ileus
- Mental obtundation (can range from full alertness to coma)
- Tachypnea with air hunger (Kussmaul respirations)
- Fruity breath (caused by acetone)
- Evidence of precipitating factors (e.g., ischemia or infection)
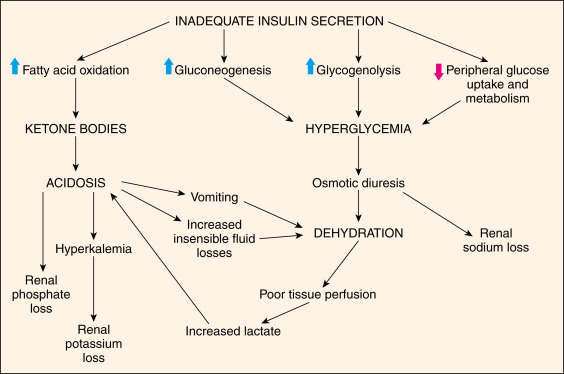
Hyperglycemia occurs from relative insulinopenia for the degree of transient insulin resistance plus an increase in counterregulatory hormones, which leads to increased hepatic gluconeogenesis and glycogenolysis. The resulting lipolysis and fatty acid oxidation produce ketonemia and metabolic acidosis. Both hyperglycemia and ketonemia result in an osmotic diuresis, which can lead to hypovolemia and subsequent decline in renal function. The pathophysiology of diabetic ketoacidosis is illustrated in Fig. E1.
DKA can be precipitated by various conditions:
- Infection (commonly of the respiratory tract, such as COVID-19, or the urinary tract or skin)
- Insulin deficiency (undiagnosed diabetes, medication nonadherence/inadequacy, insulin pump malfunction/disconnect, diabulimia)
- Inflammatory conditions (e.g., acute pancreatitis)
- Ischemia/infarction (e.g., myocardial infarction, stroke, bowel ischemia)
- Severe extracellular fluid volume depletion
- Drugs (e.g., steroids; thiazides; atypical antipsychotics; SGLT2 inhibitors; alcohol; sympathomimetics, including cocaine; and cancer treatment involving immune checkpoint inhibitors)