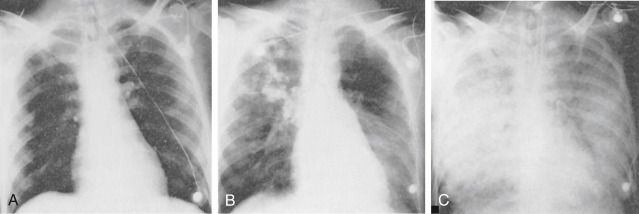
Nonpharmacologic TherapyTreatment of ARDS is supportive. There is no specific pharmacotherapy for ARDS. Management principles are summarized in Table 4.
TABLE 4 ARDS Management Principles
| Supportive Care |
| Supplemental oxygen to ensure adequate oxygenation |
Lung-protective ventilation
- Volume and pressure limited
- Ensure ventilator synchrony
- Prone position if PaO2/FiO2 <150 despite protective ventilation
|
| Reduce oxygen consumption if hypoxia is critical |
| Support adequate perfusion for other organs; focus on both cardiac output and blood pressure |
| Find and Treat Underlying Cause |
| Consider infections, mimics |
| Minimize Further Edema Accumulation |
| Seek lowest pulmonary microvascular pressure that maintains adequate perfusion |
| Diurese/reduce vascular volume while maintaining adequate perfusion |
| Avoid Harm |
| Volume- and pressure-limited ventilation strategy |
| Avoid both hypotension and volume overload |
| Goal-directed sedation with frequent reassessment |
| Avoid hyperoxia |
| Consider early physical rehabilitation |
| Seek and treat neuromuscular, cognitive, and psychological impairments during recovery |
ARDS, Acute respiratory distress syndrome; FiO2, fractional concentration of oxygen in inspired gas; PaO2, partial pressure of arterial oxygen.
From Broaddus VC et al: Murray & Nadel’s textbook of respiratory medicine, ed 7, Philadelphia 2022, Elsevier.
Hemodynamic monitoring:
- Can be used for the initial evaluation of ARDS (in ruling out cardiogenic pulmonary edema) and its subsequent management. However, a pulmonary catheter is not indicated in the routine management of ARDS. Trials have shown that clinical management involving the early use of pulmonary artery catheters in patients with ARDS did not significantly affect mortality and morbidity rates and may result in more complications as compared with a central venous catheter.
- Although no dynamic profile is diagnostic of ARDS, the presence of pulmonary edema, a high cardiac output, and a low pulmonary capillary wedge pressure (PCWP) is characteristic of ARDS.
- It is important to remember that partially treated intravascular volume overload and flash pulmonary edema can have the hemodynamic features of ARDS; filling pressures can also be elevated by increased intrathoracic pressures or with fluid administration; cardiac function can be depressed by acidosis, hypoxemia, or other factors associated with sepsis.
Ventilatory support:
- Noninvasive positive-pressure ventilation (NIPPV) (i.e., BiPAP) should only be used in selected cases in patients with hypoxic respiratory failure.
- A randomized multicenter, open-label trial showed that high-flow oxygen by nasal cannula increased ventilator-free days and reduced 90-day mortality compared with NIPPV in patients with hypoxemic respiratory failure without hypercapnia. Either modality should not delay intubation and mechanical ventilation initiation in patients with rapidly progressing clinical deterioration.
- Mechanical ventilation is generally necessary to maintain adequate gas exchange. Ventilatory strategy for patients with ARDS as proposed by the ARDS Network is summarized in Fig. 2. A low tidal volume and low plateau pressure ventilator strategy are recommended to avoid ventilator-induced injury. Assist-control is generally preferred initially with the following ventilator settings:
- FiO2 1.0 (until a lower value can be used to achieve adequate oxygenation). When possible, minimize oxygen toxicity by maintaining FiO2 at <60%.
- Tidal volume: Set initial tidal volume at 6 ml/kg of predicted body weight (PBW). Tidal volumes are reduced from 6 mL/kg of PBW to a minimum of 4 mL/kg if plateau airway pressures exceed 30 cm of water. The concept of using PBW is based on the fact that lung size depends most strongly on height and sex; PBW normalizes the tidal volume to lung size. Aim to maintain plateau pressure (Pplat) at <30 mm Hg PEEP 5 cm H2O or greater (to increase lung volume and keep alveoli open).
- PEEP should be increased in small increments of 3 to 5 cm H2O to achieve acceptable arterial saturation (>0.9) with nontoxic FiO2 values (<0.6) and acceptable airway plateau pressures (<30 to 35 cm H2O). It is important to remember that an increase in PEEP may lower cardiac output and, despite improvement in PaO2, may actually have a negative effect on tissue oxygenation (the major determinants of tissue oxygenation are hemoglobin, percent saturation, and cardiac output). The optimal level of PEEP remains unestablished.7 Although higher levels of PEEP may help prevent life-threatening hypoxemia and be associated with lower hospital mortality in patients meeting criteria for ARDS, such benefit is unlikely in patients with less severe lung injury (PaO2/FiO2 >200) and a strategy of treating such patients with high PEEP levels may be harmful. A study published in 2017 demonstrated that the open lung approach increases mortality in patients with moderate to severe ARDS.8
- Inspiratory flow: 60 L/min.
- Ventilatory rate: High ventilatory rates of up to 35 breaths/min are often necessary in patients with ARDS to achieve the desired minute ventilation because of their increased physiologic dead space and smaller lung volumes. Patients must be monitored for excessive intrathoracic gas trapping (auto-PEEP or intrinsic PEEP) that can depress cardiac output.
- Permissive hypercapnia: To maintain a low plateau pressure, a low tidal volume is frequently required, leading to a reduced minute ventilation and hypoventilation with consequently a respiratory acidosis (elevated PCO2 and reduced pH). Most patients (excluding patients with cerebral edema, acute coronary syndrome, seizures, cardiac arrhythmias, and so on) can tolerate a low pH without major consequences. Bicarbonate replacement is suggested when the pH falls to below 7.20.
- Sedation: Gamma-aminobutyric acid (GABA) receptor agonists (including propofol and benzodiazepines) have traditionally been the most commonly administered sedative drugs for ICU patients. Recent trials indicate that the alpha-2 agonist dexmedetomidine (Precedex) may have distinct advantages. At comparable sedation levels, dexmedetomidine-treated patients spent less time on ventilator, experienced less delirium, and developed less tachycardia and hypertension. The most notable adverse effect of dexmedetomidine was bradycardia.
- Neuromuscular blockade: The benefits of early continuous neuromuscular blockade in patients with ARDS who are receiving mechanical ventilation remains unclear. In a trial among patients with moderate-to-severe ARDS who were treated with a strategy involving a high PEEP, there was no significant difference in mortality at 90 days between patients who received an early and continuous infusion of the neuromuscular blocking agent cisatracurium and those who were treated with a usual-care approach with lighter sedation targets.9,10
- Discontinuing ventilation/extubation: Fig. 3 is an algorithm for assessing whether a patient is ready to be liberated from mechanical ventilation and extubated.
Figure 3 Algorithm for Assessing Whether a Patient is Ready to Be Liberated from Mechanical Ventilation and Extubated
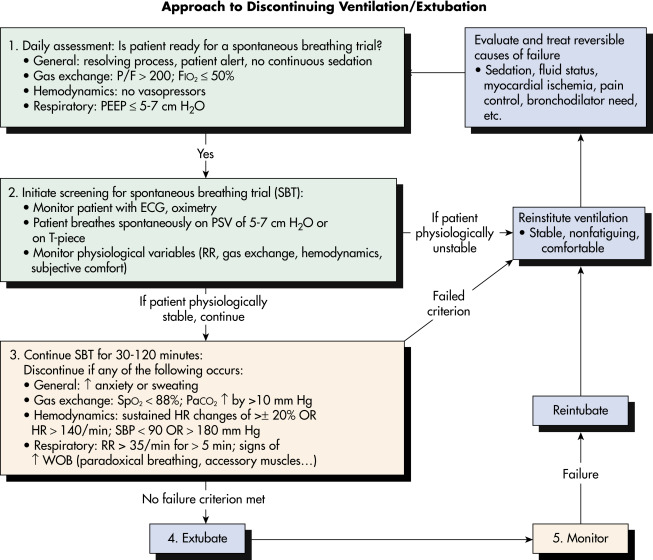
ECG, Electrocardiogram; HR, heart rate; PaCO2, arterial partial pressure of carbon dioxide; PEEP, positive end-expiratory pressure; P/F, PaO2/FiO2; PSV, pressure support ventilation; RR, respiratory rate; SBP, systolic blood pressure; SpO2, oxygen saturation based on pulse oximeter; WOB, work of breathing.
From Goldman L, Shafer AI: Goldman-Cecil medicine, ed 26, Elsevier, 2020.
Figure 2 Ventilatory Strategy for Patients with the Acute Respiratory Distress Syndrome (ARDS) as Proposed by the ARDS Network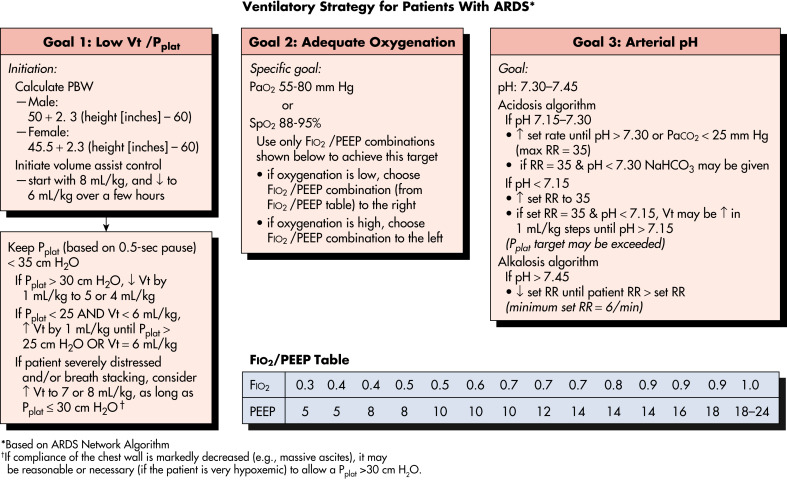
Several caveats should be considered in using the low tidal volume strategy. (1) Tidal volume (VT) is based on predicted body weight (PBW), not actual body weight; PBW tends to be about 20% lower than actual body weight. (2) The protocol mandates decreases in the VT lower than 6 mL/kg of PBW if the plateau pressure (Pplat) is greater than 30 cm H2O and allows small increases in VT if the patient is severely distressed or if there is breath stacking, as long as Pplat remains at 30 cm H2O or lower. (3) Because arterial carbon dioxide (CO2) levels will rise, pH will fall; acidosis is treated with increasingly aggressive strategies dependent on the arterial pH. (4) The protocol has no specific provisions for the patient with a stiff chest wall, which in this context refers to the rib cage and abdomen; in such patients, it seems reasonable to allow Pplat to increase to more than 30 cm H2O, even though it is not mandated by the protocol; in such cases, the limit on Pplat may be modified on the basis of analysis of abdominal pressure, which can be estimated by measuring bladder pressure. FiO2, Fraction of inspired oxygen; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PBW, predicted body weight; Pplat, plateau pressure; PEEP, positive end-expiratory pressure; RR, respiratory rate; SpO2, oxygen saturation based on pulse oximeter; VT, tidal volume.
From Goldman L, Shafer AI: Goldman-Cecil medicine, ed 26, Elsevier, 2020.
Acute General RxIdentify and treat precipitating conditions:
- Blood and urine cultures and trial of antibiotics in presumed sepsis (routine administration of antibiotics in all cases of ARDS is not recommended).
- Prompt repair of bone fractures in patients with major trauma.
- Crystalloid resuscitation in pancreatitis.
- Fluid management: In most patients with ARDS, fluid restriction is associated with better outcomes than a liberal fluid policy. Optimal fluid and hemodynamic management of patients with ARDS should be patient specific; in general, administration of crystalloids is recommended if a downward trend in PCWP is associated with diminished cardiac index, resulting in prerenal azotemia, oliguria, and relative tachycardia.11
- Positioning the patient: Changes in position can improve oxygenation by improving the distribution of perfusion to ventilated lung regions; repositioning (lateral decubitus positioning) should be attempted in patients with hypoxemia that is not responsive to other medical interventions. Placing patients with moderate and severe hypoxemia in a prone position may improve their oxygenation. A meta-analysis that included the recent trials by Guerin et al12 have shown that in patients with severe ARDS, early application of prolonged (over 16 h/day) prone-positioning sessions significantly decreases 28-day and 90-day mortality.
- Corticosteroids: Routine use of corticosteroids in ARDS is not recommended; corticosteroids may be beneficial in patients with many eosinophils in the bronchoalveolar lavage fluid or in patients with severe pneumonia. Systemic infections should be ruled out or adequately treated before administration of corticosteroids. Use of methylprednisolone has not been shown to increase the rate of infectious complications but is associated with a higher rate of neuromuscular weakness. In addition, starting methylprednisolone therapy more than 2 wk after the onset of ARDS may increase the risk of death.
- Nutritional support: Nutritional support, preferably administered by the enteral route, is necessary to maintain adequate colloid oncotic pressure and intravascular volume. The use of antioxidants and dietary oil supplements is still equivocal and cannot be recommended at this time.
- Tracheostomy: Tracheostomy is warranted in patients requiring >2 wk of mechanical ventilation; discussion regarding tracheostomy should begin with patient (if alert and oriented) and/or family members/legal guardian after 5 to 7 days of ventilatory support. Early tracheostomy (within 4 days of admission to critical care) does not limit mortality and results in many unneeded procedures.12
- Some form of deep vein thrombosis prophylaxis is indicated in all patients with ARDS.
- Stress ulcer prophylaxis with sucralfate suspension (by nasogastric tube), or proton pump inhibitors (PO or IV) or histamine-2 blockers (PO or IV). Should be reserved for seriously ill patients who are at high risk for this complication.13
- The use of surfactant remains controversial. Patients who receive surfactant have a greater improvement in gas exchange in the initial 24-h period than patients who receive standard therapy alone; however, the use of exogenous surfactant does not improve survival.
- Fig. 4 illustrates the management of acute respiratory failure from acute lung injury and ARDS.
- The SARS-CoV-2 virus first identified in 2019 can lead to severe ARDS. There was debate on whether ARDS caused by the virus merited special management. There is now consensus and sufficient evidence that it should not be treated differently than other ARDS resulting from other etiologies. (See chapter on viral pneumonia.)14,15
Figure 4 Management of Acute Respiratory Failure from Acute Lung Injury and Acute Respiratory Distress Syndrome
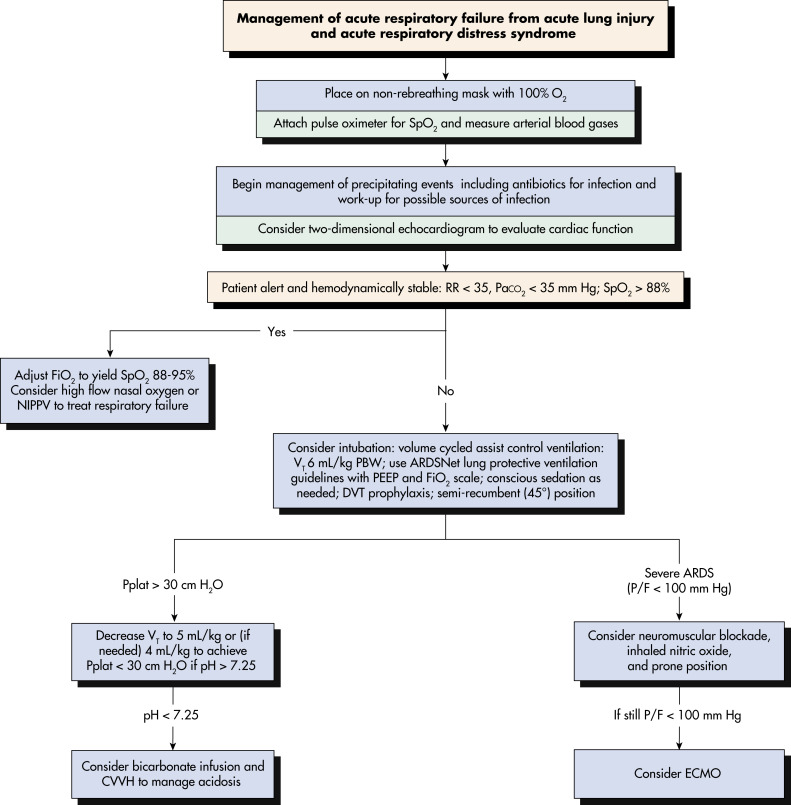
CVVH, Continuous venovenous hemofiltration; DVT, deep venous thrombosis; ECMO, extracorporeal membrane oxygenation; NIPPV, noninvasive positive pressure ventilation; PBW, predicted body weight; PEEP, positive end-expiration pressure; P/F, PaO2/FiO2; Pplat, plateau airway pressure; RR, respiratory rate; SpO2, arterial oxygen saturation; VT, tidal volume.
From Goldman L, Shafer AI: Goldman-Cecil medicine, ed 26, Elsevier, 2020.
ReferralSurgical referral for tracheostomy (see “Acute General Rx”).
Referral to ECMO team or center in severe cases when indicated.