AUTHOR: Fred F. Ferri, MD
Hirsutism is the development of stiff, pigmented (terminal) facial and body hair (male distribution) in women (Fig. E1) as a result of excess androgen production.
- Overall prevalence unknown, estimated 5% to 10% in reproductive age women.
- Race and genetics should be considered. Some distinct ethnic populations have minimal body hair and others (Mediterranean, Middle Eastern, South Asian) have moderate to large amounts of body hair while serum androgen levels are similar.
- Social norms and culture also determine how much body hair is cosmetically acceptable.
- Half of all cases of mild hirsutism do not have hyperandrogenemia. “Patient-important hirsutism” refers to hirsutism causing woman sufficient distress to seek care.
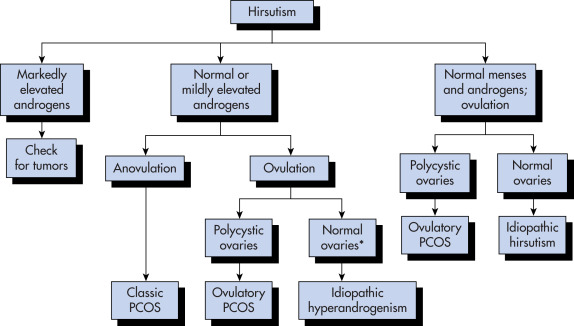
- Incidence and presentation of hirsutism is dependent on underlying cause of androgen excess (see “Differential Diagnosis”).
- Most women with hirsutism have polycystic ovary syndrome (PCOS). PCOS accounts for 95% of cases of hirsutism.
- Timing of symptoms: Abrupt onset, short duration, rapid progression, progressive worsening, more severe signs of virilization (Fig. E2), or later age of onset suggest androgen-producing tumor, late-onset congenital adrenal hyperplasia, or Cushing syndrome. Weight increases may produce increased androgen production.
- Menstrual history: Menarche, cycle regularity and symptoms of ovulation, fertility, and contraception use. Anovulatory cycles are the most common underlying cause of androgen excess.
- Medication use history: Some drugs cause hirsutism or produce androgenic effects (danazol, phenytoin, valproic acid, androgenic progestins [e.g., norgestrel], cyclosporin, minoxidil, metoclopramide, phenothiazines, methyldopa, diazoxide, and penicillamine).
- Family history: Known or suspected family history of hirsutism, congenital adrenal hyperplasia, insulin resistance, PCOS, infertility, obesity, menstrual irregularity may be found.
- Physical exam reveals deepening voice, body habitus, increased muscle mass, galactorrhea; abdominal and pelvic exam.
- Associated cutaneous manifestations are acne, acanthosis nigricans, striae, hair distribution, location and quantity, frontotemporal balding, muscle mass, clitoromegaly.
- Ferriman-Gallwey scale, a simple, pictorial system of scoring nine body areas, is the most common tool used to quantify hirsutism. It may be unreliable in non-Caucasian women of other ethnicities.
- Presence of hirsutism indicates androgen excess. Total testosterone may be normal, but free testosterone is elevated.
- Androgens induce vellus hair follicles (soft, unpigmented hair) in sex-specific areas (upper lip, chin, midsternum, upper abdomen, back, buttocks) to develop into thicker, more heavily pigmented terminal hairs.
- Anovulatory ovaries are usual source of excess androgens through thecal cell steroidogenesis and conversion of androstenedione to testosterone. The most common cause of hirsutism is polycystic ovary syndrome, which accounts for three out of every four cases.
- Conditions that decrease hepatic production of sex hormone binding globulin (SHBG) decrease protein-bound testosterone and increase free testosterone fraction (e.g., low estrogen, high androgen, and hyperinsulinemic states).
- Late-onset, congenital adrenal hyperplasia enzyme deficiency (most commonly 21-hydroxylase deficiency) produces excess 17 hydroxyprogesterone (17-OHP) and overproduction of androstenedione.
- Rare ovarian tumors primarily derived from Sertoli-Leydig cells, granulosa theca cells, or hilus cells produce excess androgens.
- Rare adrenal tumors produce excess androgens.
- Rare pituitary or hypothalamic tumors produce excess prolactin and can lead to anovulation.
- Box E1 summarizes causes of androgen excess in women of reproductive age.