AUTHOR: Bharti Rathore, MD
Thyroid carcinoma is a primary neoplasm of the thyroid and consists of four major subtypes: Papillary, follicular, anaplastic, and medullary. A classification of thyroid neoplasms is described in Table E1.
TABLE E1 Classification of Thyroid Neoplasms
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2019, Elsevier.
Papillary carcinoma of thyroid
Follicular carcinoma of thyroid
Anaplastic carcinoma of thyroid
Medullary carcinoma of thyroid
| ||||||||||||||||||||
- Thyroid cancer is the most common endocrine cancer, with an estimated 43,800 new cases and 2230 deaths occurring in 2022 in the U.S.
- Incidence is 13.9 per 100,000 people in the U.S. and increasing over last 4 decades.
- Female:male ratio is 3:1.
- Median age at diagnosis: 45 to 50 yr.
- Occult thyroid cancer is identified in 20% of autopsy specimens.
- Thyroid cancer is often identified incidentally.
- Physical exam may reveal:
- Presence of thyroid nodule
- Hoarseness and cervical lymphadenopathy
- Painless swelling in the region of the thyroid
- Risk factors: Prior neck irradiation.
- Multiple endocrine neoplasia II (medullary carcinoma).
- Inherited syndromes associated with thyroid cancer are described in Table 2.
- GLP-1 receptor agonists for the treatment of type 2 DM (e.g., exenatide, albiglutide) can increase the risk of medullary thyroid carcinoma (MTC).
- Papillary thyroid carcinoma is the commonest type of thyroid carcinoma of follicular origin and encompasses several tumor types that have mutually exclusive mutations that activate thyroid cell abnormal proliferation. BRAF V600E mutation accounts for 60% of these mutations; other mutations include RAS or RET/PTC rearrangements.1,2
- In follicular thyroid carcinoma, oncogenic drivers are primarily RAS alterations (40% to 50% of cases) and PAX8/PPARγ rearrangements (30% to 40% of cases). Molecular alterations of the PI3K/Akt pathway and PTEN silencing by inactivating mutations or epigenetic changes also occur in some cases.
- Poorly differentiated thyroid carcinomas are aggressive cancers with a high mutation rate. RAS and BRAF mutations are found in 20% to 50% and up to 35% of cases, respectively. Genetic alterations that characterize PDTC and are associated with tumor aggressiveness are TERT promoter (20% to 50%) mutations and TP53 mutations (10% to 35%) that cooccur with RAS and BRAF mutations.1,2
- In anaplastic thyroid cancer, as many as 25% to 50% of cases harbor BRAFV600 mutations, which can be targeted with available BRAF/MEK inhibitor combination therapy. Other frequent mutations include TERT (75%), TP53 (63%), and RAS (24%). Other actionable molecular drivers with available targeted therapies include RET rearrangements, ALK rearrangements, NTRK fusions, and TSC2 mutations, suggesting the need for broad molecular profiling.
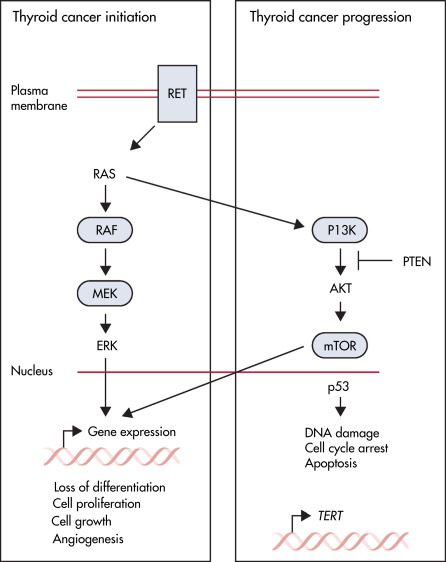
- Pathways in the development of thyroid cancer are depicted in Fig. E1.
TABLE 2 Inherited Syndromes Associated With Thyroid Cancer
| Multiple endocrine neoplasia (MEN) 2A and 2B | |||
| Isolated familial medullary thyroid cancer | |||
| Gardner syndrome | |||
| Familial adenomatous polyposis | |||
| Carney complex | |||
| Cowden syndrome | |||
| Familial nonmedullary thyroid cancer |
From Cameron JL, Cameron AM: Current surgical therapy, ed 10, Philadelphia, 2011, Saunders.
Figure E1 Thyroid cancer pathways.
Diagram shows the key molecular signaling pathways involved in thyroid cancer. The box on the left shows the mitogen-activated protein kinase pathway, which is activated by mutation in most thyroid cancers. These events are believed to initiate thyroid cancer development and lead to altered gene expression, which promotes cell proliferation, cell growth, angiogenesis, and loss of differentiation. The box on the right shows pathways altered in advanced thyroid cancers, which are believed to promote tumor progression. This includes the PI3K-mTOR pathway, the p53 tumor suppressor, and alterations in the promoter for TERT. Blue boxes represent factors for which targeted treatments are available that have been approved by the U.S. Food and Drug Administration. mTOR, Mammalian target of rapamycin; PI3K, phosphatidylinositol-3-kinase; TERT, telomerase reverse transcriptase.
From Cabanillas ME et al: Thyroid cancer, Lancet 388[10061]:2783-2795, 2016.