AUTHORS: Harikrashna B. Bhatt, MD and Russell E. Bratman, MD



DefinitionMultiple endocrine neoplasia (MEN) refers to a group of heritable genetic syndromes characterized by the development of specific groups of tumors of the endocrine glands.
SynonymsMEN I: Wermer syndrome
MEN IIA: Sipple syndrome
| ICD-10CM CODES | | E31.2 | Multiple endocrine neoplasia [MEN] syndromes | | E31.20 | Multiple endocrine neoplasia [MEN] syndrome, unspecified | | E31.21 | Multiple endocrine neoplasia [MEN] syndrome type I | | E31.22 | Multiple endocrine neoplasia [MEN] syndrome type IIA | | E31.23 | Multiple endocrine neoplasia [MEN] syndrome type IIB |
|
Epidemiology & Demographics 12Incidence
- MEN I: 25 in 10,000
- MEN II: 1 in 30,000
Prevalence
- MEN I: 1/30,000
- MEN II: 1/35,000 (predominantly MEN IIA)
Risk FactorsFamily history of MEN syndrome, although it can also occur sporadically
Genetics
- MEN I: Autosomal-dominant mutation in MEN1 tumor-suppressor gene1
- MEN IIA and MEN IIB: Autosomal-dominant mutations in RET proto-oncogene2
Physical Findings & Clinical Presentation
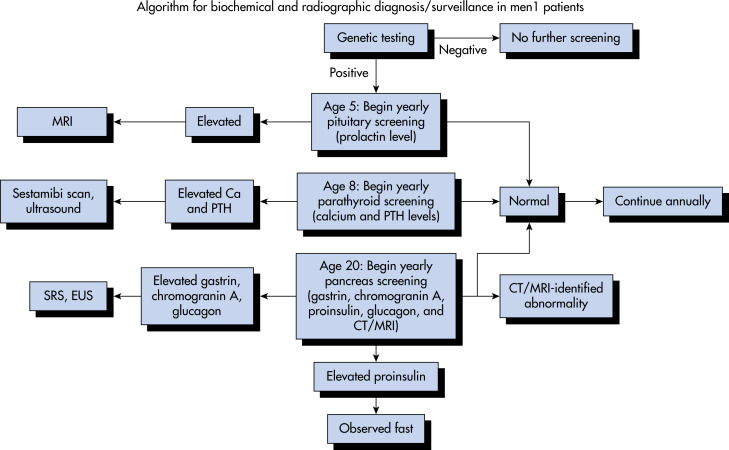
- Patients may present due to screening or may present with a MEN-associated tumor. Tumors are found incidentally due to biochemical abnormalities or to symptoms.
- MEN I (PPP [pituitary, pancreas, parathyroid])3,4
- Diagnostic criteria generally include two MEN-associated tumors, one tumor in a patient with a family history, or positive genetic testing
- Primary hyperparathyroidism (parathyroid adenoma or hyperplasia) is the most common manifestation and can cause hypercalcemia (urolithiasis, GI disturbance, bone pain, neuropsychiatric disturbances) and also affect bone density
- Pancreatic neuroendocrine tumors cause symptoms related to their secretory properties or metastases
- Gastrinoma: Peptic ulcers, diarrhea, esophageal symptoms (see “Gastrinoma”)
- Insulinoma: Hypoglycemic symptoms upon fasting and after exercise (see “Insulinoma”)
- Glucagonoma: Necrolytic migratory erythema (a blistering skin lesion), diabetes/glucose intolerance, weight loss
- VIPoma: Diarrhea, hypokalemia, decreased gastric acid
- Somatostatinoma: Hyperglycemia, cholelithiasis, diarrhea, abdominal pain, weight loss
- Nonfunctioning tumors can metastasize (frequently to the liver), a frequent cause of death in MEN I
- Pituitary tumors may cause compressive symptoms, such as visual field defects or hypopituitarism (see “Pituitary Adenoma”). Hormonal secretion may also cause symptoms.
- Prolactinoma (most common pituitary tumor in MEN I): Menstrual irregularities, hypogonadism, gynecomastia, and/or galactorrhea (see “Prolactinoma”)
- Somatotroph adenomas (producing growth hormone): Gigantism or acromegaly (frontal bossing, increased shoe/hat size, hyperglycemia, hyperhidrosis) depending on patient age (see “Acromegaly”)
- Corticotroph adenomas (producing adrenocorticotropic hormone): Cushingoid features such as weight gain, moon facies, hyperglycemia, bone loss, proximal muscle weakness, hypertension, hypokalemia (see “Cushing Disease and Syndrome”)
- Nonfunctioning (nonsecretory) tumors
- Other manifestations: Carcinoid tumors, collagenomas, angiofibromas, meningiomas, lipomas
- MEN IIA:5,6
- Medullary thyroid carcinoma (MTC) is a tumor of the thyroid’s calcitonin-secreting C cells. It can present with a neck mass, as well as flushing and diarrhea due to elevated calcitonin levels.
- Primary hyperparathyroidism: See “MEN I.” Less aggressive in “MEN IIA.”
- Pheochromocytoma is an adrenal tumor producing catecholamines, which can lead to life-threatening hypertensive crises.
- MEN IIB:5,6
- MTC
- Pheochromocytoma
- Oral mucosal neuromas
EtiologyTumor development facilitated by the previously described genetic mutations (Table E1).
TABLE E1 Tumors in Multiple Endocrine Neoplasia Syndromes
| Type (Chromosomal Location) | Tumors (Estimated Penetrance) |
|---|
| MEN 1 (11q13) | Parathyroid adenoma (90%) |
| Enteropancreatic tumor (30%-70%)
Gastrinoma (40%)
Insulinoma (10%)
Nonfunctioning and PPoma (20%-55%)
Glucagonoma (<1%)
VIPoma (<1%) |
| Pituitary adenoma (30%-40%)
Prolactinoma (20%)
Somatotropinoma (10%)
Corticotropinoma (<5%)
Nonfunctioning (<5%) |
| Associated Tumors
Adrenal cortical tumor (40%)
Pheochromocytoma (<1%)
Bronchopulmonary NET (2%)
Thymic NET (2%)
Gastric NET (10%)
Lipomas (30%)
Angiofibromas (85%)
Collagenomas (70%)
Meningiomas (8%) |
| MEN 2 (10 cen- 10q11.2) | |
| MEN 2A | MTC (90%)
Pheochromocytoma (50%)
Parathyroid adenoma (20%-30%) |
| MTC only | MTC (100%) |
| MEN 2B (also known as MEN 3) | MTC (>90%)
Pheochromocytoma
Associated abnormalities (40%-50%)
Mucosal neuromas
Marfanoid habitus
Medullated cornel nerve fibers
Megacolon |
MEN, Multiple endocrine neoplasia; MTC, medullary thyroid carcinoma; NET, neuroendocrine tumor; PPoma, pancreatic polypeptidoma; VIPoma, vasoactive intestinal peptidoma.
Modified from Thakker RV: Multiple endocrine neoplasia type 1. In Jameson JL et al (eds): Endocrinology: adult and pediatric, Philadelphia, 2016, Saunders.

No therapy available at this time to reverse the underlying genetic cause. Treatment (both medical and surgical) focuses on tumor prevention and management.
Nonpharmacologic Therapy
- MEN I:3,4
- For hyperparathyroidism, surgical parathyroidectomy is indicated in patients with significant hypercalcemia, osteoporosis, renal disease, or nephrolithiasis or found to be at high risk for nephrolithiasis. Hyperparathyroidism may be treated surgically with parathyroidectomy.
- Pituitary tumors may be surgically excised via transsphenoidal approach; notably, medical therapy is first line for prolactinoma.
- Pancreatic tumor treatment is extremely variable. Gastrinoma is frequently complicated by duodenal metastases, which are difficult to treat surgically, so treatment choice varies between centers. Ulcers caused by gastrinoma may require endoscopic or surgical management. Surgery is preferred for insulinoma, VIPoma, and glucagonoma. Nonfunctioning tumors may be treated surgically depending on tumor size and location. Conservative management for insulinoma may involve frequent carbohydrate intake. Cytotoxic chemotherapy and tyrosine kinase inhibitors are options for patients with metastatic disease. Peptide receptor radionucleotide therapy (PRRT) is an emerging and exciting therapeutic modality for metastatic disease.
- MEN IIA:
- MTC is generally treated surgically. Prophylactic thyroidectomy is considered based on genetic mutation. Almost all children with MEN IIA will require thyroidectomy (screening as previously described). Patients should undergo ultrasound and total thyroidectomy with cervical lymph node dissection. Extent of further neck dissection depends on metastases and may be guided by calcitonin levels. A more palliative surgical approach is considered in the setting of advanced disease.5,6
- Hyperparathyroidism: Resection of only enlarged glands with intraoperative PTH monitoring is preferred.
- Pheochromocytoma is treated surgically. Unilateral adrenalectomy is preferred, although many patients will develop a contralateral pheochromocytoma. Preoperative blood pressure control with alpha blockade is key. If present, pheochromocytoma must be removed before thyroidectomy.7
- MEN IIB:
- MTC: Prophylactic thyroidectomy offered in childhood.5,6
- Pheochromocytoma: See “MEN IIA.”
Acute General Rx
- MEN I:3,4
- Hyperparathyroidism causes hypercalcemia that may be treated with intravenous (IV) hydration, diuretics, and bisphosphonates. Early vitamin D repletion prevents bone destruction postoperatively due to the “hungry bone syndrome.”
- Gastrinoma may lead to peptic ulcers requiring IV proton-pump inhibitor (PPI).
- Supportive care (glucose for hypoglycemia caused by insulinoma; fluids and electrolytes for hypovolemia caused by diarrhea from VIPoma or gastrinoma) is required.
- MEN IIA:
- Hyperparathyroidism: See “MEN I.”
- Pheochromocytoma: Preoperative blood pressure control is key; alpha blockers are first line. Phenoxybenzamine irreversibly blocks alpha adrenergic receptors; doxazosin may also be helpful. Calcium channel blockers can be utilized. There is concern that unopposed beta blockade may allow for alpha-medicated vasoconstriction.7
- MEN IIB:
- Pheochromocytoma: See “MEN IIA.”
Chronic Rx
- MEN I:3,4
- Hyperparathyroidism may be amenable to agonists of the calcium-sensing receptor (cinacalcet), although surgery is preferred in patients who meet surgical criteria and are operative candidates.
- Pancreatic tumors may benefit from medical therapy.
- Gastrinoma: PPI or H2 antagonists, somatostatin agonists (such as octreotide)
- Insulinoma: Diazoxide or somatostatin agonists
- Glucagonoma and VIPoma: Somatostatin agonists
- Adjunctive medical therapy can be considered for pituitary tumors, although surgical treatment is first-line with the exception of prolactinoma (for which dopamine agonists are first line). Radiation therapy and/or medical therapy are considered in cases of incomplete resection or regrowth.
- Prolactinoma: Dopamine agonists (bromocriptine or cabergoline). Temozolomide is a chemotherapeutic agent for refractory cases.
- Somatotroph adenoma: Somatostatin or dopamine agonists, growth hormone receptor antagonist
- Corticotroph adenoma: Antiadrenal agents, adrenal enzyme blocker (such as ketoconazole), somatostatin or dopamine agonists, glucocorticoid receptor blockers
- MEN II A:
- Hyperparathyroidism: See “MEN I.”
- MTC: Tyrosine kinase inhibitors and chemotherapy may help treat metastatic disease. After thyroidectomy, levothyroxine is indicated (thyroid-stimulating hormone suppression not required).5,6
- MEN IIB
MTC: See “MEN II A.”
DispositionMost workup can be done as an outpatient (multidisciplinary team); inpatient stays may be required due to acute complications or for surgical procedures.
Complementary & Alternative MedicineMEN is unlikely to be amenable to this.
ReferralPatients with MEN should be followed by a multispecialty team, including endocrinologists, endocrine surgeons, and genetic counselors.