AUTHOR: Fred F. Ferri, MD


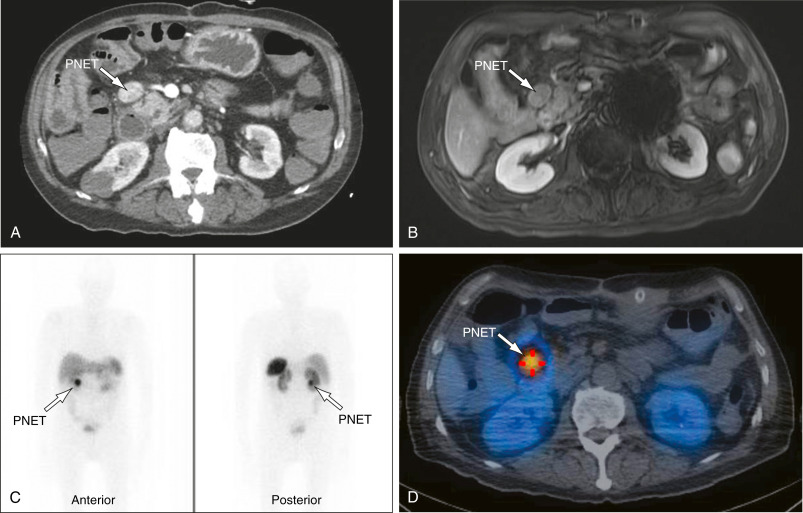
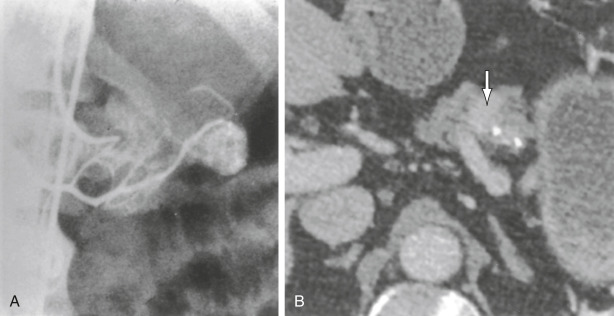
DefinitionInsulinoma is a pancreatic insulin-secreting tumor that leads to inappropriately elevated plasma insulin or proinsulin levels with suppression of hepatic glucose output and subsequent hypoglycemia, especially during periods of fasting.
| ICD-10CM CODES | | C25.4 | Malignant neoplasm of endocrine pancreas | | D13.7 | Benign neoplasm of endocrine pancreas | | D37.7 | Neoplasm of uncertain or unknown behavior of other digestive organs (pancreas) |
|
Epidemiology & DemographicsIncidenceOne case per 250,000 persons annually. 90% of insulinomas are benign.
Predominant Sex & AgeInsulinomas occur in both sexes (approximately 60% in women) and at all ages. In a Mayo Clinic series, the median age at diagnosis was 50 yr in sporadic cases, but 23 yr in patients with multiple endocrine neoplasia, type 1 (MEN-1).
Physical Findings & Clinical PresentationSymptoms typically occur in the morning before breakfast (i.e., fasting hypoglycemia as opposed to reactive hypoglycemia, which is not commonly associated with insulinoma).
Etiology, Pathology, & Pathophysiology
- Insulinomas are almost always solitary. Malignant insulinomas account for 5% of the total; they tend to be larger (6 cm). Metastases are usually to the liver (47%), regional lymph nodes (30%), or both.
- Insulinomas are evenly distributed in the head, body, and tail of the pancreas; ectopic insulinomas are rare (1% to 3%). Tumor size: 5% are ≤0.5 cm, 34% are 0.5 to 1 cm, 53% are 1 to 5 cm, and 8% are >5 cm.
- Histologic classification includes insulinoma in 86% of patients, adenomatosis in 5% to 15%, nesidioblastosis in 4%, and hyperplasia in 1%. Adenomatosis consists of multiple macroadenomas or microadenomas and occurs especially in patients with MEN-1. Nesidioblastosis is also a diffuse lesion in which islet cells form as buds on ductular structures.